Introduction
Vestibular neuritis (VN) presents as an acute spontaneous whirling-type dizziness caused by sudden unilateral vestibular dysfunction [1]. However, labyrinthitis, sudden hearing loss with dizziness, and neurolabyrinthitis may present similar symptoms and signs as VN. Therefore, the term acute unilateral vestibulopathy (AUV) is recommended instead of VN to define all pathologies involving a sudden decrease of unilateral peripheral vestibular function regardless of the exact location of the lesion. On the other hand, acute vestibular syndrome (AVS) is an upper-level entity that includes both AUV, which is the most common cause of AVS (up to 70%), and acute central vertigo [usually posterior circulation stroke (PCS)]. AVS typically presents with the symptoms of new onset continuous vertigo, motion intolerance, gait instability, and nausea/vomiting lasting several days to weeks [2-5].
The Head Impulse, Nystagmus, and Test of Skew (HINTS) battery test [including a head impulse test (HIT), examination of gaze-evoked nystagmus, and test of skew deviation] is well known as an easily available bedside examination that can better differentiate AUV from central vertigo at the early stage compared to diffusion weighted MRI [6]. Among the tests included in the HINTS battery, horizontal HIT has been established in previous reports as a simple and useful test for evaluating the vestibule-ocular reflex (VOR) with high sensitivity.
The recent development and application of a video head impulse test (vHIT) device have led to great improvements in the differential diagnosis and treatment of AVS for patients with dizziness at both clinics and emergency centers. The device has several advantages over bedside HIT including the ability to record saccades, calculate gains, observe covert saccades, and test the vertical canals. Compared to the caloric test, which is the golden standard test for evaluating VOR, vHIT is less uncomfortable for the patient and requires a shorter period of time [4,5,7]. Therefore, the test can be easily performed as a first vestibular function test at clinics for dizzy patients. Our aim is to review the effectiveness of vHIT for the clinical diagnosis and treatment of AVS.
Differential diagnosis
AVS, which refers to a higher level concept than AUV, includes both central and peripheral dizziness. Although the most common cause of AVS is VN, PCS may present similar symptoms and signs as VN. In clinics, the simple examinations included in the HINTS battery are more sensitive than early MRI [6,8,9]. Among the three conditions tested in HINTS examinations, mild skew deviation is rare, but when detected, it strongly suggests a central lesion. Gaze-evoked nystagmus is also observed in only about 50% of cerebellar strokes, but strongly suggests a central problem. HIT is the most important test for central and peripheral differentiation, suggesting a central lesion when negative. A corrective saccade in the vHIT strongly suggests a peripheral lesion, but anterior inferior cerebellar artery (AICA) infarction may also show abnormal vHIT. If other neurologic signs such as hearing loss, facial palsy, ataxia, or sensory deficit are accompanied with abnormal vHIT, than AICA infarction should be suspected in patients with high risk factors of infarction. Therefore, the diagnosis should be confirmed using other neurological examinations [4,8,10].
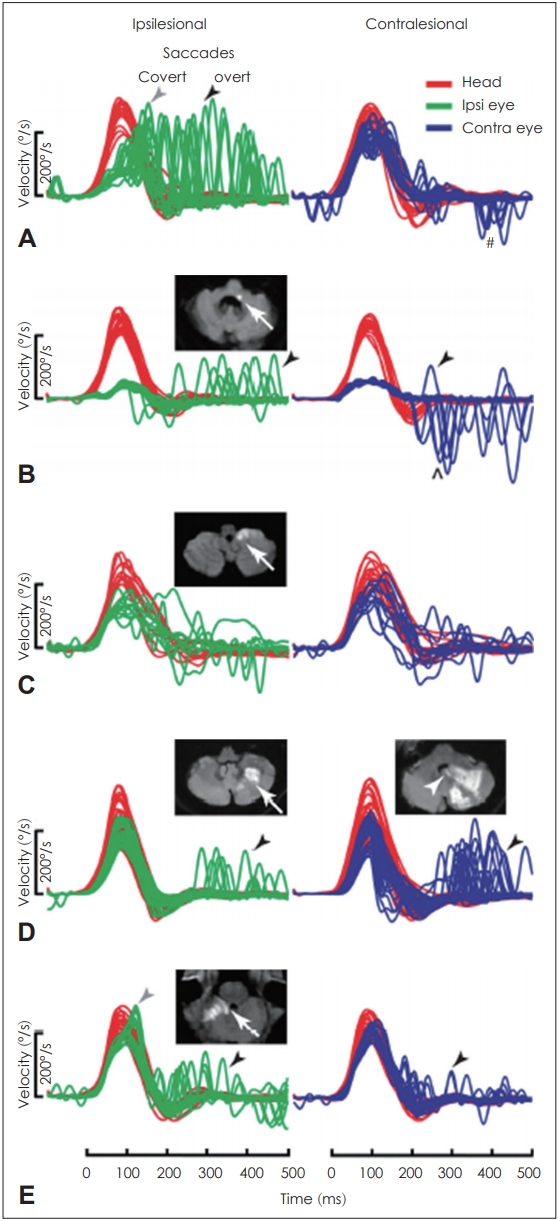
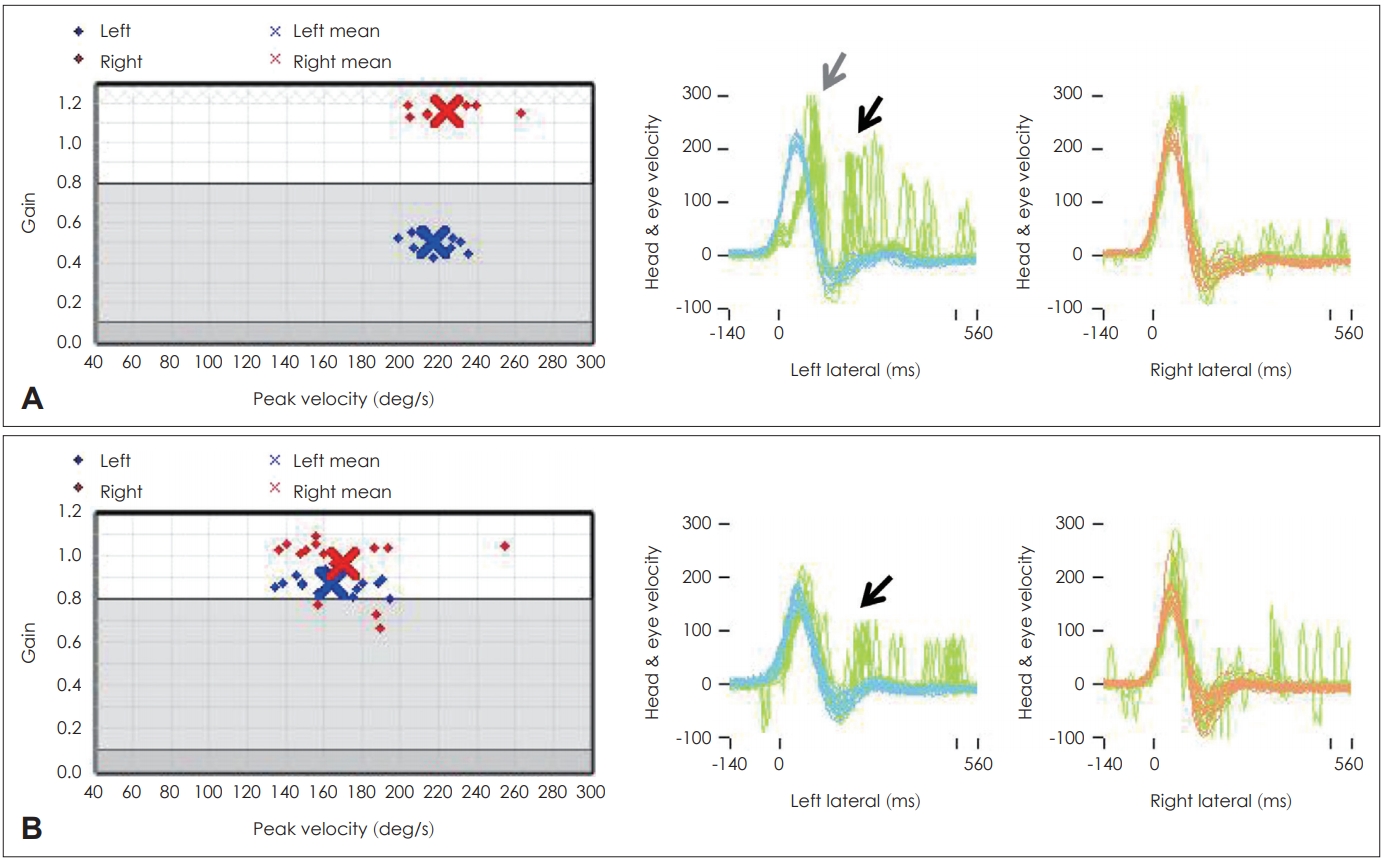
A bedside HIT, directly observed with the eyes, has several disadvantages: it is difficult to observe the covert saccades that occur when the head moves, it is highly dependent on the proficiency of the examiner; and it is difficult to evaluate quantitatively. The vHIT can be used in emergency departments or outpatient clinics as an alternative to compensate for these disadvantages for the differentiation of AVS [11,12]. The gain of the vHIT can be used to differentiate VN from PCS with 88% sensitivity and 92% specificity, which is more accurate than early diffusion-weighted image MRI [10,13]. According to Mantokoudis, et al. [14], a lateral semicircular canal gain over 0.7041 was reported as an optimal discrimination cut-off point for stroke. In cases of PCS infarction, when the inner ear is involved in AICA infarction, gain reduction can be observed on the lesion side, but if the vestibular nucleus is damaged, symmetrical gain reduction with small catch-up saccades can be seen on both sides. In cases of posterior inferior cerebellar artery (PICA) infarction, there is a slight symmetrical gain reduction on both sides and small catch-up saccades (Fig. 1, Table 1) [12]. However, it has been reported that the gain of vHIT can vary in cases of anterior cerebellar infarction, thus, differential diagnosis only based on the gain of vHIT can be risky. Clinicians must also consider AICA infarctions during differential diagnosis despite positive results in bilateral vHIT, as they are not always attributable to peripheral lesions [11,12,15].
In addition, unlike bedside HIT, which is directly seen with the eyes, artifacts can occur during vHIT testing (like slippage of the goggles); therefore, clinicians should avoid mistakes that can occur when performing a diagnosis based on the normal range of the gain provided by the vHIT [3]. The value of the gain can significantly vary according to the algorithms and the type of vHIT device used [16]. In addition to the absolute value of the gain, the canal function can also be determined using bilateral gain asymmetry, as in cases of canal paresis determined with the bithermal caloric test: gain asymmetry (GA)=(right gain - left gain)/(right gain + left gain)×100%. The normal range of GA has not been established yet, but it can be considered abnormal if it is 8% or greater regardless of age [17-19]. Furthermore, even if the gain is normal, it is also important to differentiate abnormal saccades, since they can appear in normal function and can have similar shapes in spontaneous nystagmus and eye blinking [20]. According to a previous report, when the peak velocity of the corrective saccade exceeds 100˚/s and when the peak velocity difference between the ears is more than 40˚/s, the saccadic movement can be defined as abnormal [17,18]. However, if the peak velocity criteria are used for the determination of abnormality, the given head impulse velocity is very important and should not be too high or too low (typically 150-250˚/s).
When a patient visits with acute vertigo symptoms consistent with VN and unilateral sudden onset hearing loss for the 1st attack presentation, the clinical diagnosis could be ‘sudden hearing loss with vertigo’ or ‘labyrinthitis’ after the exclusion of AICA infarction. In a previous study using vHIT on patients with sudden hearing loss with vertigo, vHIT showed abnormalities most often in the posterior semicircular canal (74% of patients), and the frequency was much higher than the 41% observed in the lateral canal and the 30% observed in the superior canal. The involvement of the posterior canal in sudden hearing loss with vertigo has a different pattern from that of typical VN and is thought to be caused by focal infarction of the common cochlear artery, which supplies the cochlea and posterior semicircular canal [3,21,22]. In a study evaluating the recovery of vestibular function in patients with Ramsay-Hunt syndrome with vertigo and those with VN using vHIT, patients with VN recovered significantly faster than those with Ramsay-Hunt syndrome [23].
Subtypes of VN
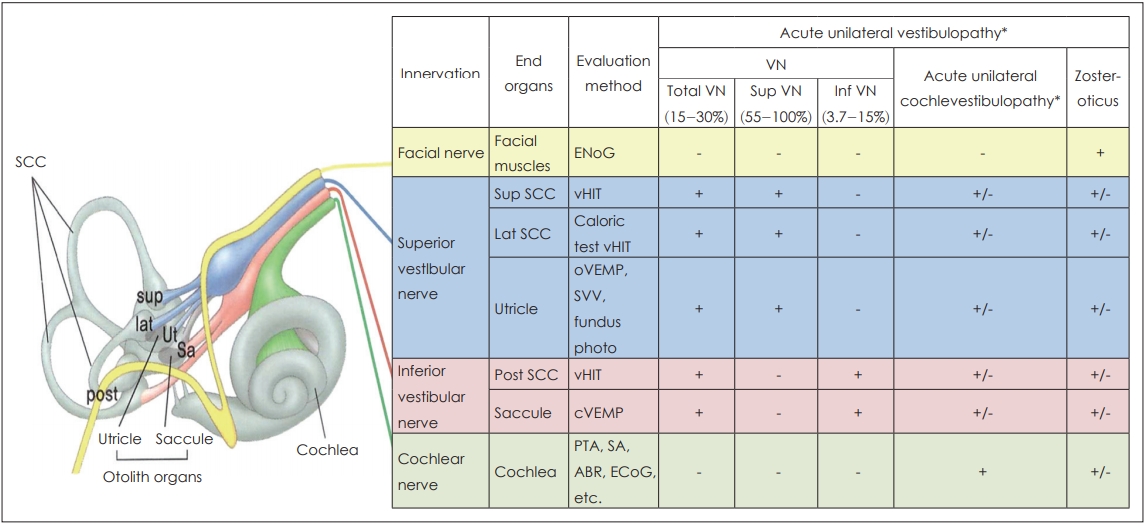
As mentioned earlier, rather than evaluating abnormalities in the lateral semicircular canal based on catch-up saccades with a bedside HIT, quantitative evaluation of semicircular canal functions are now possible using vHIT as well as the evaluation of each vertical canal. In addition, the subtypes of VN can be distinguished using the cervical vestibular-evoked myogenic potential (cVEMP) that measures the function of the saccule (inferior vestibular nerve), ocular vestibular myogenic potential (oVEMP) that measures the function of the utricle (superior vestibular nerve), subjective visual vertical test, and fundus photography (Fig. 2) [24-26]. There have also been reports of different types of AUV that invade only one canal or one vestibular organ in the superior or inferior vestibular nerve branch. This condition may also be called ampullary VN [20,24,27].
Superior VN
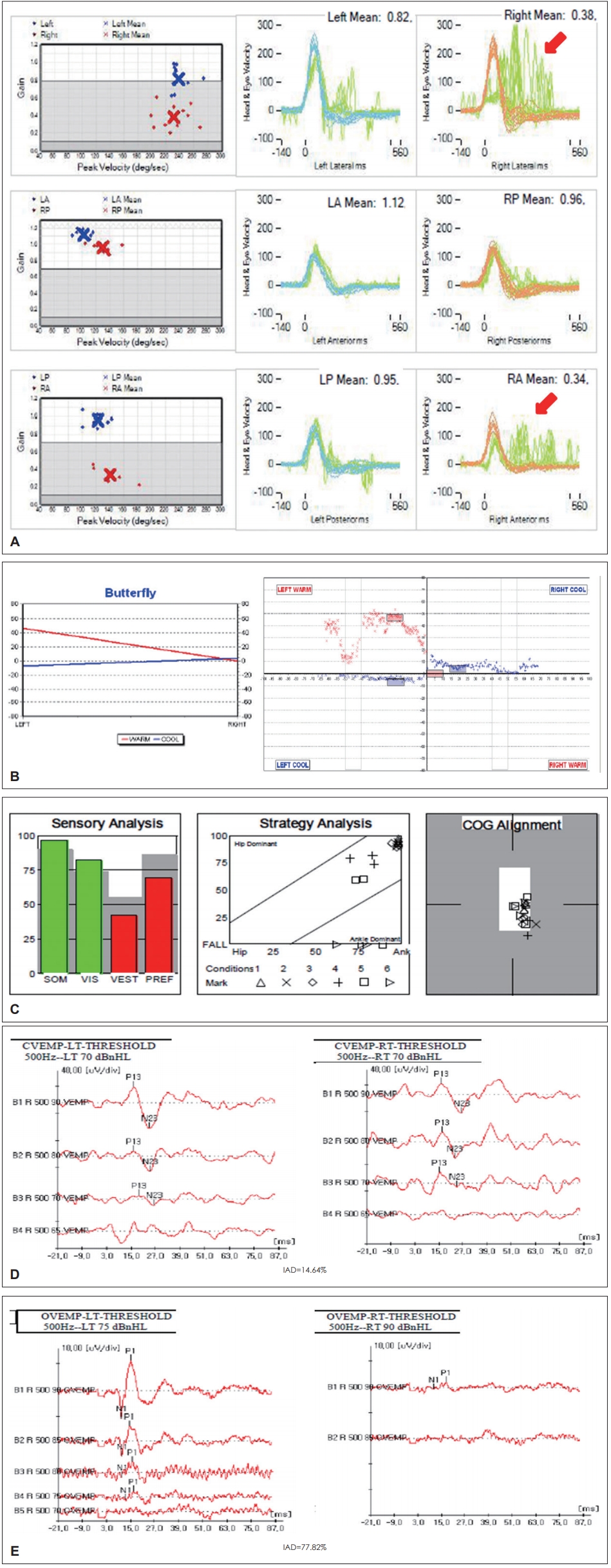
The most common (>50%) type of AUV is superior VN. In general, superior VN shows canal paresis in the caloric test with abnormal findings in oVEMP and normal cVEMP results. In vHIT for superior VN, the gain in the ipsilateral lateral canal and superior canal is reduced and catch-up saccade can be observed (Fig. 3). In the literature, there are a few reports of isolated lateral canal involvement in vHIT reported for VN patients but no reports of isolated superior canal involvement [20,25].
Inferior VN
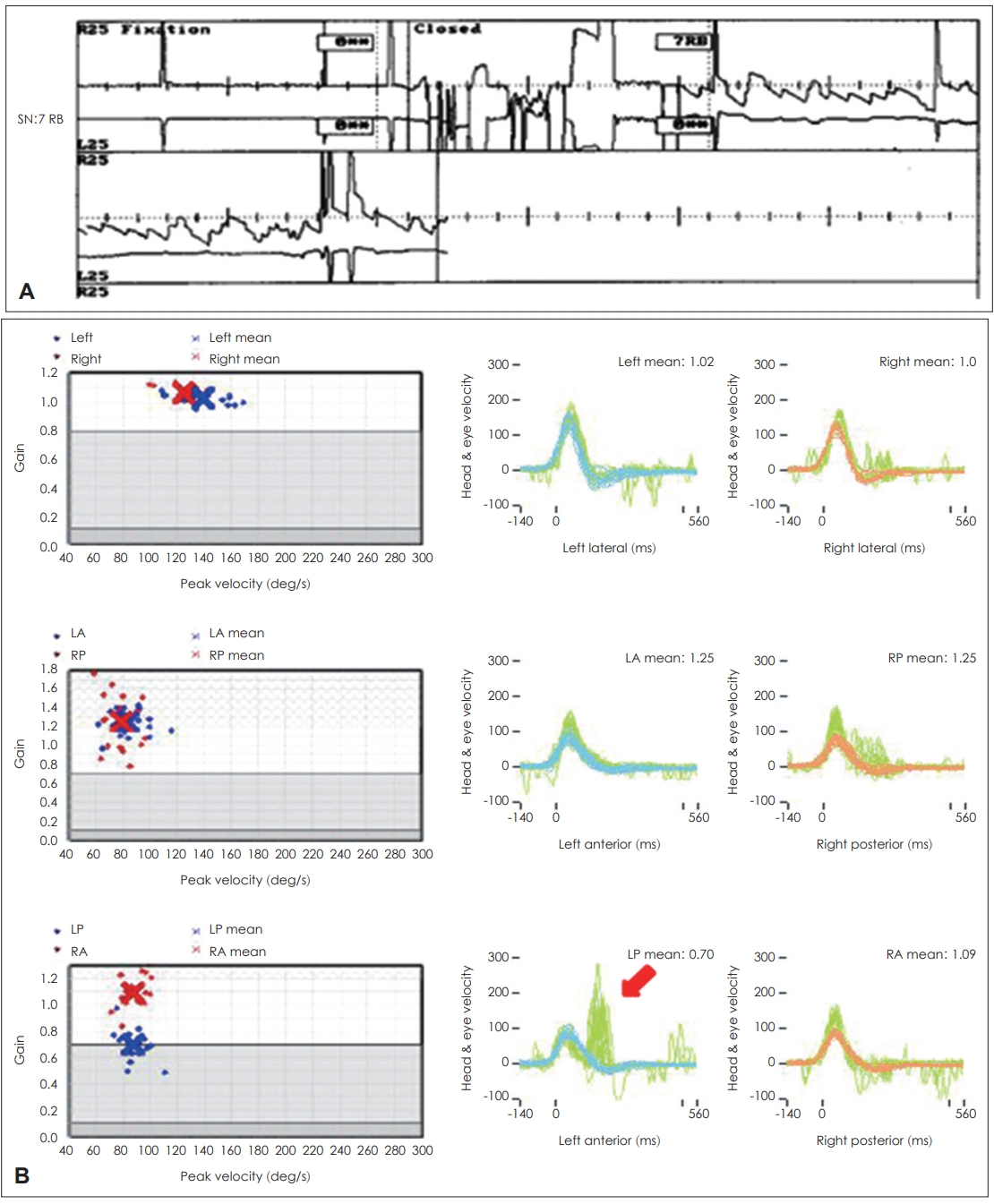
In inferior VN, a relatively rare (2% to 15%) type of VN, vHIT shows abnormalities in only the posterior semicircular canal with normal findings in the lateral and superior canals (Fig. 4) [28]. Additionally, vestibular dysfunction is observed on the cVEMP. In a typical case of inferior VN, spontaneous nystagmus cannot be seen with a normal caloric function test; hence, this type of VN can be misinterpreted as a central lesion. In this case, vHIT can help distinguish between central and peripheral lesions based on abnormalities in the posterior canal [12,29]. Recently, in the study by Park, et al. [29] on the correlation between oVEMP and the posterior canal of vHIT in inferior VN, the correlation was relatively lower than predicted. This may be because the inferior vestibular nerve is divided into the saccular nerve and posterior ampullary nerve, and thus both tests can be used to complement each other [30].
Clinical course and follow-up
The vHIT can be used to evaluate rapid vestibular ocular reflexes (2-6 Hz) closer to normal head movements (0.1-5 Hz) compared to the conventional caloric test (0.002 Hz) and the rotatory chair test (0.01-0.64 Hz) [31]. In addition, the vHIT has a shorter test time than the other tests and causes less inconvenience to the patient. Therefore, it is possible and useful to repeat the test several times for each patient during the follow-up period to validate the treatment results. In a recent study using vHIT and other vestibular function tests to determine the efficacy of steroids in VN, there was no evidence that steroid therapy was effective for the treatment of VN. This finding was in contrast with previous reports using a caloric test that showed the effectiveness of steroids [32].
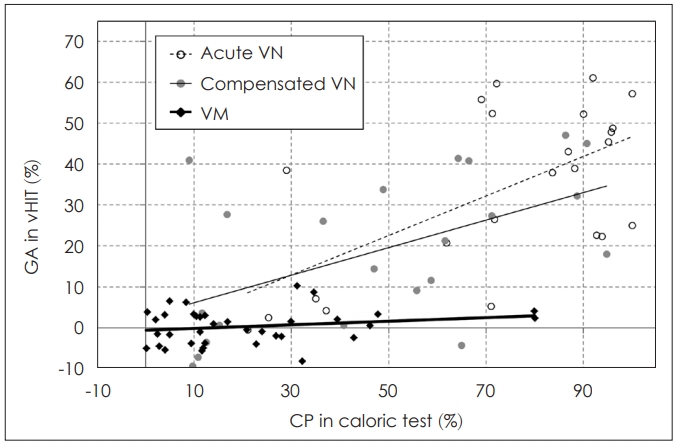
The discrepancies between the evaluation of the lateral semicircular canal function using the vHIT and canal paresis in the caloric test are relatively well known, especially in Meniere’s disease. The discrepancies in Meniere’s disease are explained by two theories; first, as we know, there are type I and type II hair cells, and type II hair cells (which usually does not response to high frequency stimulus) are more frequently affected in Meniere’s disease, so vHIT can be frequently preserved in Meniere’s disease (cell type theory); second is ‘hydropic labyrinth theory,’ physical enlargement of the membranous labyrinth in Meniere’s disease would allow heated endolymph circulate within the canal instead of generating a thermally induced pressure across the cupula. Thus, the cupula deflection will be diminished, so reducing the response to caloric stimulation [7,20,33-35]. However, compared to Meniere’s disease, there is a relatively significant correlation between the two tests in VN [35]. In a study involving patients with vestibular migraine and patients with VN, the GA of vHIT was significantly correlated with canal paresis in acute VN (R=0.35, p<0.005) as well as in chronic VN (R=0.31, p<0.01), although there was no significant correlation in the patients with vestibular migraine (Fig. 5) [19]. Since there is no proven pathophysiology of vestibular migraine and diagnosis is done based on symptoms, this disagreements between two tests in vestibular migraine are difficult to explain but it is presumed that there are frequency-dependent vestibular dysfunction in vestibular migraine resulting different vHIT and caloric results [35,37]. So both vHIT and caloric test should be combined to evaluate patient’s semicircular canal function for broader spectrum of stimulating frequencies.
In the clinical course of VN, the normalization rate of the caloric test is known to be 40-66% after 6 months to 1 year [17,23,32,34,38,39]. In the case of vHIT, the normalization rate was reported to be higher than that of the caloric test [17]. In other studies, the normalization rate of the lateral canal gain in the lesion was 38-52% at 1 month after onset and 79-87% at 6 months after onset using vHIT [1,17,40]. However, both the gain (abnormal gain <0.8) and the saccade abnormality (abnormal saccade >100˚/s) were significantly more abnormal (Fig. 6; at 1 month, 29% when only the gain was analyzed and 13% when the saccade was analyzed with the gain) [17,41]. According to Büki, et al. [1], vertical canals had a lower recovery rate than lateral canals and the lowest recovery rate was observed when only the posterior canal was involved. In contrast, Magliulo, et al. [26] reported that when vestibular function was evaluated in combination with VEMP, regions in the superior vestibular nerve showed a slower recovery of function. This finding was explained by the earlier recovery of the utricular and saccular nerves followed by the ampullary nerve [27]. When a decrease in the vHIT gain or canal paresis was severe in the acute stage, the recovery of the gain was found to be significantly lower after this stage [42]. In the study by Cerchiai, et al. [43], patients with significantly lower vHIT gain in the early stage had a significantly higher dizziness handicap index (DHI) score at 1 month and vestibular rehabilitation was necessary for these patients.
Symptom improvement during the recovery process for VN varies for each patient. In some cases of VN, the symptom is long-lasting and changes in the symptoms do not always coincide with the results of the vestibular function test [44]. The rate of improvement (normalized to within 10) of DHI in VN has been reported to be approximately 64% to 87% [27,32]. However, the association between the severity of dizziness symptoms and vHIT gain has been reported differently in studies. Patel, et al. [44] found that the severity of dizziness evaluated using DHI and the Vertigo Symptoms Scale short-form in patients with chronic stage VN was not significantly correlated with the results of vHIT.
Conclusion
The vHIT is relatively simple to perform, has a short test time, and has the advantages of quantitatively evaluating each of the semicircular canals. Recently, vHIT equipment was widely adopted in many institutions in Korea. This device can be useful for differential diagnosis of patients with AUV including VN. In addition, it can be useful for AUV classification according to the extent of VN involvement, prediction of prognosis, and identification of treatment progress.