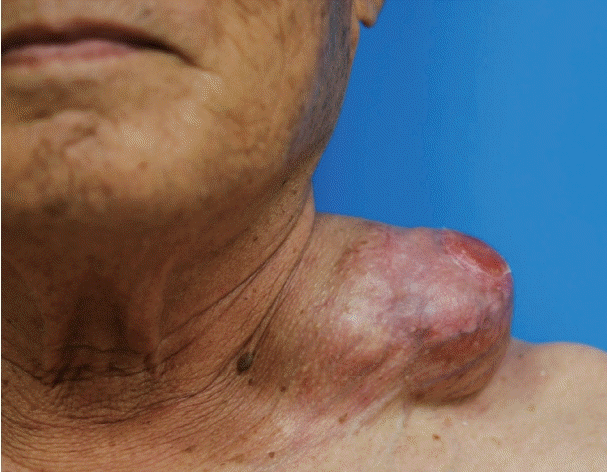
경부에 발생한 거대한 저등급 섬유점액성 육종
Giant Low-Grade Fibromyxoid Sarcoma in the Neck
Article information
Trans Abstract
Low-grade fibromyxoid sarcoma (LGFS) is a soft tissue tumor that rarely occurs in the head and neck region. It occurs mainly in the proximal extremities and the trunk and is prevalent in the young and middle-aged adults. In the present case, LGFS was present at an atypical location and at an unusual age. The treatment of choice for LGFS is radical wide surgical excision with a clear margin. Long-term follow-up is essential for all patients with LGFS, as it has the potential for late recurrence or metastasis.
Introduction
Low-grade fibromyxoid sarcoma (LGFS) is a rare and soft tissue tumor and was first reported by Evans in 1987 [1-5]. LGFS is characterized by the benign histologic appearance and very indolent but fully malignant behavior [1-9]. Most commonly it arises in the lower extremities; however, the occurrence in the head and neck region is considered to be extremely rare [1-9]. Herein, we report a case of giant LGFS of the left neck region with multiple recurrences.
Case
A 78-year-old male visited our hospital with an enlarged, painless mass in the left neck region that had lasted for more than 40 years. The patient had undergone several surgeries for the removal of the mass in the same area of the neck. Two years back, the size of the tumor had increased suddenly. Physical examination revealed 9 cm sized huge mass with superficial skin ulceration in the left supraclavicular area (Fig. 1). CT scans revealed a 9×8 cm sized well-defined multi-lobulated heterogeneously enhancing mass in the left supraclavicular area (Fig. 2). The preoperative fine-needle aspiration cytology showed mixed spindle cells and inflammatory components.
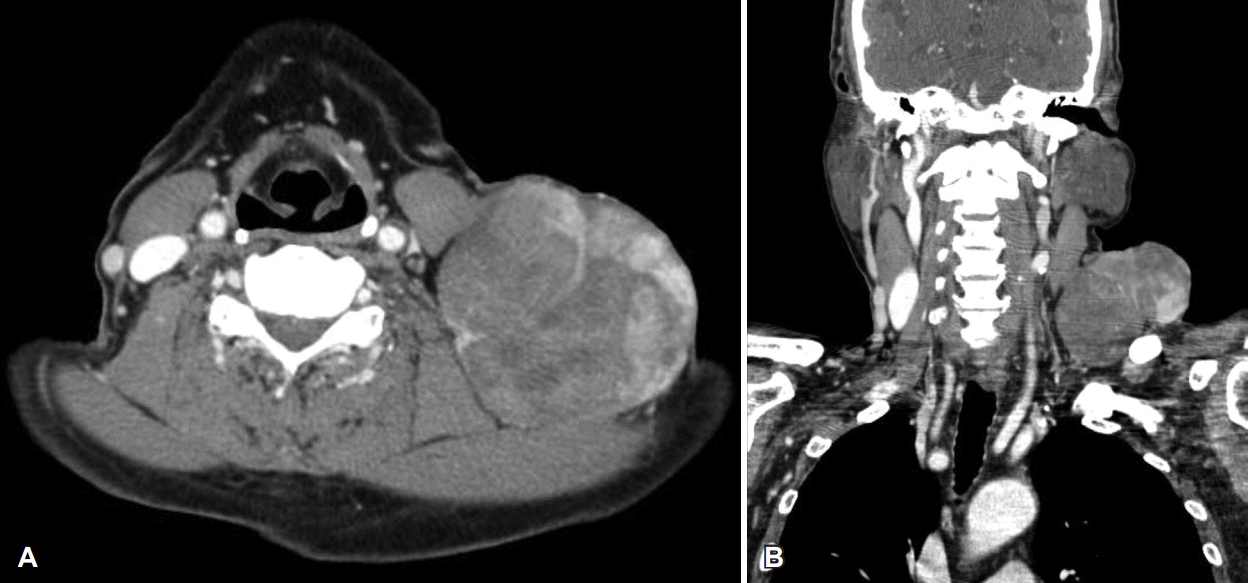
Axial (A) and coronal (B) CT scans demonstrate a 9×8 cm sized well-defined multi-lobulated heterogeneously enhancing mass in the left supraclavicular area.
Left supraclavicular mass wide resection with clear margin including skin was performed under general anesthesia (Fig. 3). The mass was relatively well separated from the surrounding tissues and had no involvement of the brachial plexus and phrenic nerve. Surgery was completed after primary closure of the skin. The postoperative course was uneventful. The histopathologic examination of left supraclavicular mass was diagnosed as LGFS (Fig. 4). The patient is continuously being observed from the past one year without recurrence or metastasis.

Surgical specimen (A). Photo of surgical field after tumor removal (B). SCM: Sternocleidomastoid muscle.
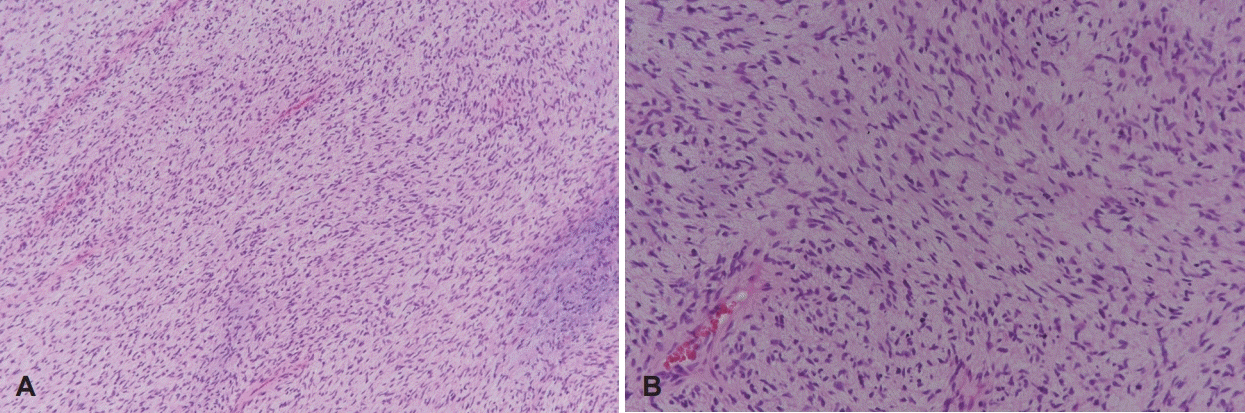
Slides reveal an admixture of collagenized zones and myxoid zones with prominent capillary-sized blood vessels. The tumor cells are bland spindled cells with short fascicular and whorling growth patterns (H&E stain, ×100) (A). The tumor cells are bland spindled cells with short fascicular and whorling growth patterns (H&E stain, ×200) (B). H&E stain: hematoxylin and eosin staining.
Discussion
LGFS represents approximately 10% of soft tissue sarcoma and is rarely found in the head and neck region [1-5,7,9]. It occurs mainly in the proximal extremities and trunk and is prevalent in the young and middle-aged adults [1-7]. In the present case, LGFS occurred at an atypical location and at an unusual age.
The presenting symptom of LGFS is a painless, slow-growing, non-tender, firm mass, similar to the symptoms observed in our patient [2,3]. CT and MRI are helpful to detect the extent of LGFS and determine the treatment plan [2,3,9].
However, the final diagnosis of LGFS is only made by the histopathologic examination [1-8]. LGFS shows low to the moderately cellular tumor with whirling pattern of tumor cells, composed of alternating myxoid and collagenous area [1-5]. Pericollageneous rosettes with cells around a collagenous center can also be seen [1,2]. This case was diagnosed with LGFS due to the appearance of bland spindled cells with short fascicular and whorling growth patterns in the tumor. Immunohistochemistry of LGFS is non-characteristic and shows only constant positive for vimentin and occasional positive for CD34 [2,3]. Recently, MCU4 has been shown to be a sensitive and specific marker for LGFS [3,4,6]. However, there are now reports of MUC4 negative tumors with the characteristics morphological features of LGFS [7]. In this case, all of the immunohistochemistry stains, such as MCU-4, CD34, MDM2, CDK4, S-100, Actin, Desmin, Beta-catenin, were negative.
The differential diagnosis includes many benign and malignant soft tissue lesions with a variably fibrous and myxoid stroma [2-5]. LGFS can be distinguished from sarcomas with mixed components of myxoid and fibroblastic cells due to the presence of lipoblasts [2]. The differential diagnosis of LGFS includes fibromatosis, schwannoma, neurofirboma, and myxofibrosarcoma [2-5]. None of these soft tissue tumors contain collagenous rosettes which is characteristics of LGFS [2]. In addition, peripheral nerve sheath tumors, such as schwannoma, neurofirboma are positive for S100 protein and SOX10 staining, but LFGS is negative on both tests [7].
The treatment of choice of LGFS is radical wide surgical excision with clear margin [1-9]. LGFS is a low-grade tumor with low mitotic rate and is not expected to be affected by chemotherapy and radiotherapy [3-6,8]. LGFS has the potential for very late recurrence and metastasis [1-9]. A recent study on LGFS cases with long-term follow-up reported recurrence in 64%, metastasis in 45%, and death from disease in 42% cases [1,5,8]. The local recurrence interval was up to 15 years with a median of 3.5 years [1]. The interval to metastasis varied up to 45 years with a median of 5 years [1]. Distant metastasis frequently occurred in the lungs, pleura, and chest wall [1,5]. Therefore, long-term follow-up is necessary for all cases with LGFS.
In conclusion, LGFS is an extremely uncommon neoplasm of the head and neck region. Long-term follow-up is essential for all patients with LGFS, as it has the potential for late recurrence or metastasis.
Acknowledgements
None.
Notes
Author Contribution
Conceptualization: Dong Hoon Lee. Data curation: Hye Rin Lim, Jo Heon Kim, Dong Hoon Lee. Formal analysis: Dong Hoon Lee. Investigation: Hye Rin Lim, Jo Heon Kim, Dong Hoon Lee. Methodology: Hye Rin Lim, Dong Hoon Lee. Writing—original draft: Jong Min Park, Dong Hoon Lee. Writing—review & editing: all authors..