양악성 갑상선 종양에서 두경부 외과의에 의해 시행되는 고주파 소작술
Surgeon-Performed Radiofrequency Ablation for Benign and Malignant Thyroid Tumors
Article information
Trans Abstract
Radiofrequency ablation (RFA) has gained recognition as a highly effective and safe minimally invasive alternative treatment for well-selected patients with benign thyroid nodules and recurrent thyroid cancer. Despite the well-known efficacy of RFA, head and neck surgeons have had minimal involvement in both establishing clinical evidence and developing most RFA guidelines. This is partly due to the challenges surgeons face in understanding and applying these imaging-guided interventions. However, head and neck surgeons possess extensive knowledge of surgical anatomy and have significant clinical experience with the anatomical relationships of surrounding structures, which could enhance the safety and effectiveness of RFA. Their expertise allows for better management of rare but serious complications such as bleeding, airway compression, recurrent laryngeal nerve paralysis, and tumor rupture. Drawing from my experience as a head and neck surgeon with RFA, this article aims to elucidate the principles, indications, preparation, and procedure of RFA for benign and malignant thyroid tumors.
Introduction
For the past several decades, thyroidectomy has been the primary treatment for benign thyroid tumors requiring intervention, as well as for thyroid cancer. This surgical treatment involves various complications associated with the need for hospitalization, general anesthesia, scar formation, and risks such as vocal cord paralysis and hypoparathyroidism [1-4]. Since its introduction in the early 2000s, radiofrequency ablation (RFA) for thyroid tumors has been noted as a highly effective and safe minimally invasive treatment for well-selected patients with benign thyroid nodules and recurrent thyroid cancer [5-7]. According to previous studies, RFA has been shown to reduce the volume of benign thyroid nodules by 64.9% to 93.4%, leading to improvements in cosmetic concerns and compression symptoms [6-9]. In the treatment of recurrent thyroid cancer, a complete disappearance of the tumor on imaging has been reported in 68%-93% of cases and the oncological outcomes, including recurrence-free survival, have shown results comparable to those of surgery [10,11]. Thus, RFA is recognized as an effective treatment option for benign and malignant thyroid tumors, and various guidelines have been published both domestically and internationally [6,12-15]. However, in the establishment of most of these guidelines, the role of head and neck surgeons has been minimal. Additionally, from the perspective of surgeons primarily responsible for thyroid treatments, it is a reality that there are difficulties in understanding and directly applying these imaging-guided interventions. Therefore, based on my experience as a thyroid and head and neck surgeon with RFA, this article aims to introduce the principles, indications, preparation, and procedure of RFA for benign and malignant thyroid tumor.
Indications
The indications for RFA in thyroid tumors include: 1) benign thyroid nodules causing symptoms such as pain, difficulty swallowing, or a sense of a foreign body, or cosmetic concerns; 2) autonomously functioning thyroid nodules in patients who do not want or are unable to undergo surgery or radioactive iodine treatment; and 3) recurrent or metastatic thyroid cancer in patients with high surgical risk or those who refuse surgery [6,12,14,15]. The application guidelines for RFA in primary thyroid cancer are not yet clearly established. However, it can be sufficiently considered as an alternative to surgery or active surveillance in patients with papillary thyroid microcarcinoma, and as a palliative treatment when surgery is not possible. RFA is not recommended as a primary treatment for follicular tumors. For cystic nodules, ethanol ablation is recommended as the primary treatment, but RFA may be considered if symptoms persist or recur after ethanol ablation [6,12-15]. In my practice, ethanol ablation is generally performed first for completely cystic lesions. However, for partially cystic lesions that include a solid component, the author prefers RFA. By completely aspirating the cystic component before ablation and then ablating the remaining solid component and the tumor capsule, both the cystic and solid parts can be treated simultaneously.
For benign nodules, RFA should be performed only after confirming benignity through at least two ultrasound (US)-guided fine-needle aspirations or core-needle biopsies. In cases where US findings clearly suggest benignity (K-TIRADS 2) or in autonomously functioning thyroid nodules, a single benign diagnosis is sufficient. There are no absolute contraindications RFA, but caution is advised in patients with contralateral vocal cord paralysis. For pregnant women or patients with electrical devices such as pacemakers, the use of bipolar electrodes is recommended over monopolar electrodes [5,6].
Pre-Procedure Evaluation
Pre-procedure US is used to evaluate the characteristics of the thyroid nodule and its relationship with surrounding anatomical structures. The initial volume of the nodule is calculated using the formula for an ellipsoid (V=πabc/6) where a, b, and c are the three perpendicular diameters. A simplified calculation using 0.52×abc is also feasible. The volume reduction rate (VRR) is calculated as follows: VRR=(initial volume - final volume)/initial volume×100%.
Blood tests, including complete blood count, coagulation tests, thyroid function tests, and serum thyroglobulin or calcitonin levels if needed, are performed. Symptoms are self-assessed using a visual analogue scale, and cosmetic issues are evaluated using the World Health Organization cosmetic scoring system. It is essential to review any underlying conditions and medications that may increase bleeding tendencies and to evaluate vocal cord function.
Equipment Setup and Electrode Selection
The main equipment for RFA consists of the generator, cooling pump, and electrodes. The cooling pump is filled with coolant, and the generator is set for electrode type, procedure time, power output, and temperature. The types of electrodes used in RFA are categorized based on the length of the exposed electrode that generates heat through high-frequency transmission, such as 0.5 cm, 0.7 cm, 1.0 cm, 1.5 cm, 2.0 cm, etc. The size of the electrode indicates the range of ablation, roughly corresponding to the diameter of a spherical area around the electrode.
Recently, smaller electrodes of 0.38 cm have also been introduced for use with smaller lesions, though the available types of electrodes may vary depending on the equipment. The size of the electrode determines the heat transfer range during RFA, and is selected based on the size of the lesion and surrounding critical structures. Typically, electrodes of 0.5 cm are used for lesions smaller than 2 cm, 0.7 cm for lesions sized between 2-3 cm, 1 cm for lesions between 3-4 cm, and 1.5 cm or 2.0 cm for lesions larger than 4 cm [16]. The types of electrodes used in RFA are categorized based on the length of the exposed electrode that generates heat through high-frequency transmission, such as 0.5 cm, 0.7 cm, 1.0 cm, 1.5 cm, 2.0 cm, etc. The size of the electrode indicates the range of ablation, roughly corresponding to the diameter of a spherical area around the electrode.
Recently, smaller electrodes of 0.38 cm have also been introduced for use with smaller lesions, though the available types of electrodes may vary slightly depending on the equipment. The size of the electrode determines the heat transfer range during RFA, and is selected based on the size of the lesion and surrounding critical structures. Typically, electrodes of 0.5 cm are used for lesions smaller than 2 cm, 0.7 cm for lesions sized between 2-3 cm, 1 cm for lesions between 3-4 cm, and 1.5 cm or 2.0 cm for lesions larger than 4 cm [5,16].
Preparation
Patients are positioned supine with neck extension supported by a pillow. Ensure no metal objects are on the patient, and place grounding pads on the anterior thighs. For the trans-isthmic approach, consideration is given to the surgeon’s dominant hand and the desired thyroid gland orientation for the procedure. Accordingly, the US machine and RFA equipment are positioned. Typically, for right-handed operators performing procedures on the right thyroid nodules, the US machine and equipment are placed above the patient’s head, with the surgeon standing on the right side of the patient to perform the procedure (Fig. 1). Conversely, for right-handed operators performing procedures on left thyroid nodules, the US machine and equipment are positioned towards the patient’s body, with the surgeon standing near the patient’s head to perform the procedure.
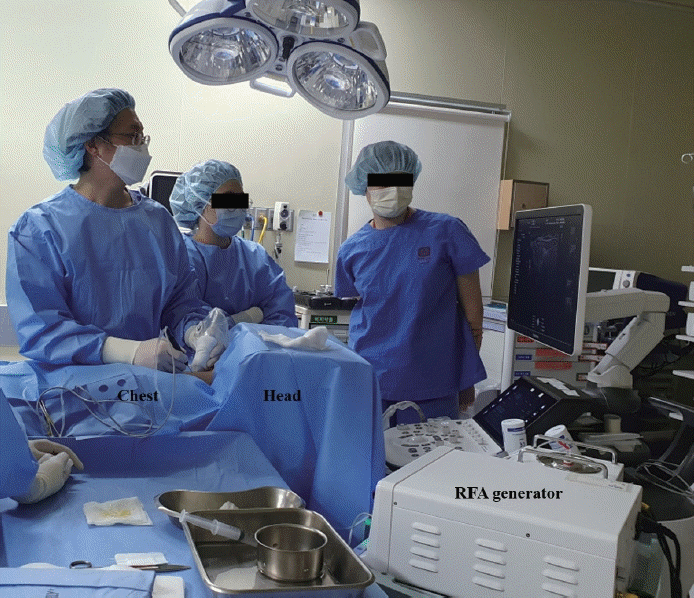
Positional setting for radiofrequency ablation. For righthanded operators performing procedures on right thyroid nodules, the ultrasound machine and equipment are placed above the patient’s head, with the surgeon standing on the patient’s right side.
Vital signs such as blood pressure, heart rate, and oxygen saturation are monitored during the procedure, and oxygen supplementation can be considered if necessary. To minimize pain and discomfort during the procedure, appropriate analgesics can be selected and administered intravenously. The procedure site is disinfected using transparent liquid disinfectants such as alcohol or chlorhexidine. Iodine-based disinfectants like povidone-iodine leave a film residue after drying, which can interfere with US transmission, and therefore are not preferred by the author. Local anesthesia is administered to the insertion site of the electrode needle and around the thyroid capsule using 1% lidocaine mixed with 1:100000 epinephrine. This helps reduce pain and can serve as a buffer space between the thyroid and surrounding structures.
Basic RFA Technique
Trans-isthmic approach and moving shot technique
The fundamental principle for inserting electrode needles in RFA is the trans-isthmic approach (Fig. 2). In this approach, electrode needle insertion starts from the isthmus of the thyroid gland and progresses in a posterolateral direction towards the target nodule, aiming to protect vulnerable structures like the recurrent laryngeal nerve and esophagus from heat generated during high-frequency ablation. This approach also minimizes the risk of high-temperature tissue fluids leaking outside the thyroid gland by passing through normal thyroid isthmic tissue between the insertion site of the electrode needle and the nodule targeted for ablation.

Schematic figure of trans-isthmic approach and moving shot technique in radiofrequency ablation. An electrode needle is inserted through the isthmus, and ablation begins from the deepest and most medial compartment of the tumor. Utilizing the moving shot technique, the tumor is continuously ablated in a unit-byunit manner. T, trachea; E, esophagus; I, internal jugular vein; C, carotid artery; V, vagus nerve.
In contrast to the static technique where the electrode needle is placed at the center of a tumor and remains stationary during ablation, the moving shot technique is employed for thyroid nodules. During this technique, the electrode needle is moved in real-time through various compartments within the nodule while ablation is ongoing. To facilitate this, pre-procedural imaging, including US, is used to three-dimensionally segment the nodule along its anterior-posterior, superior-inferior, and medial-lateral axes. These segmented regions are then ablated one by one using the moving shot technique (Fig. 2).
To start the ablation, the deepest unit of the nodule is targeted first, considering the direction of needle insertion and the artifacts produced by ablation (Fig. 3A). The electrode needle is slowly withdrawn backwards while monitoring tissue changes in real-time, abating the remaining compartments along the current path of electrode insertion. Subsequently, adjustments are made to the angle and direction of the electrode needle to treat units located more superiorly than those previously ablated (Fig. 3B). This iterative process continues, allowing for comprehensive ablation across the entire three-dimensional volume of the nodule.
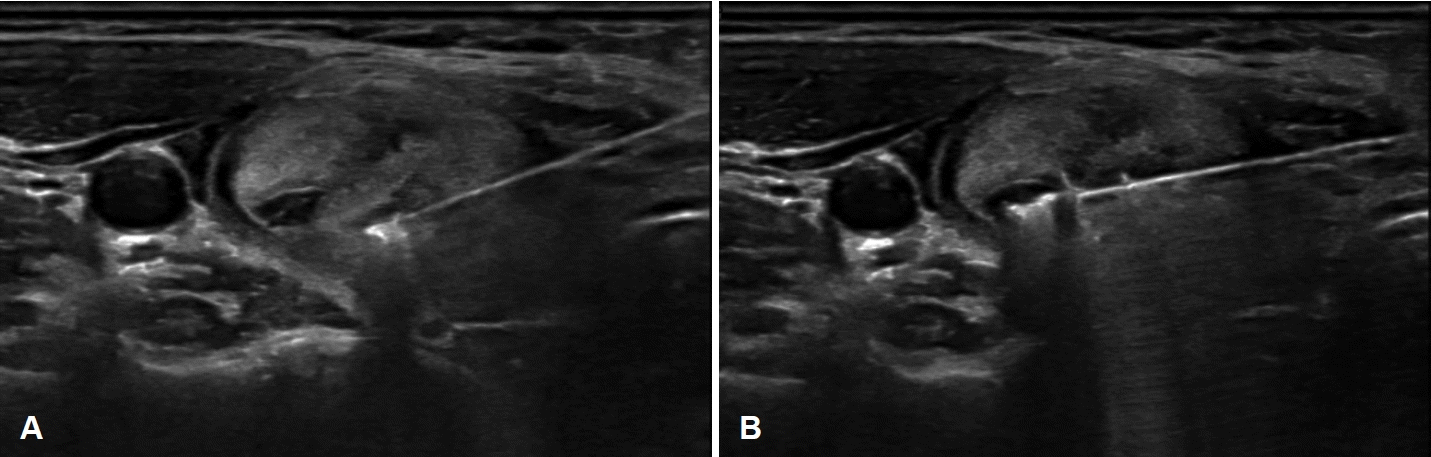
Ultrasound images of the moving shot technique. A: To minimize artifacts caused by the electrode needle and ablation, the process begins with the deepest and most medial compartment of the tumor. The electrode needle is then moved backward to ablate the remaining compartments along its insertion path. B: To treat the more superficial compartments, the insertion axis of the electrode needle is adjusted, and ablation is performed in the same manner.
Additional RFA Techniques
Hydrodissection
RFA delivers high-frequency energy through an electrode to thermally destroy tissue, making it a minimally invasive treatment method. However, its application can be challenging in lesions located in the neck, where various structures of major vessels, nerves, and organs are present in confined spaces. Hydrodissection is one of the methods to address this issue, based on the principle of injecting fluid between the tumor and surrounding critical structures to physically separate them (Figs. 4 and 5) [17-19]. The commonly used injecting solution is 5% dextrose solution. In contrast to normal saline, which is an ion-conductive fluid that can transmit electrical current generated during RFA to adjacent structures potentially causing damage, 5% dextrose solution, due to its osmotic pressure and non-ionic properties, does not conduct electricity, serving as a thermal barrier when present around target organs [17,20]. However, due to the anatomical characteristics of the neck, the injected solution can quickly disperse, potentially diminishing the separation effect. Therefore, continuous injection of the solution may be necessary to maintain sufficient safe space for hydrodissection.
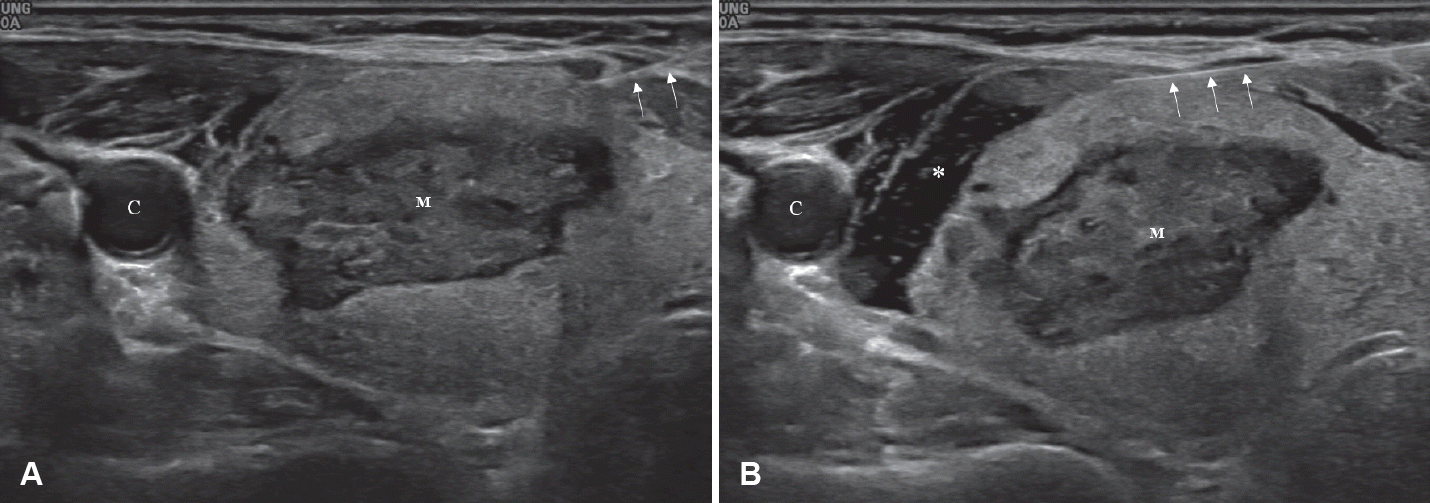
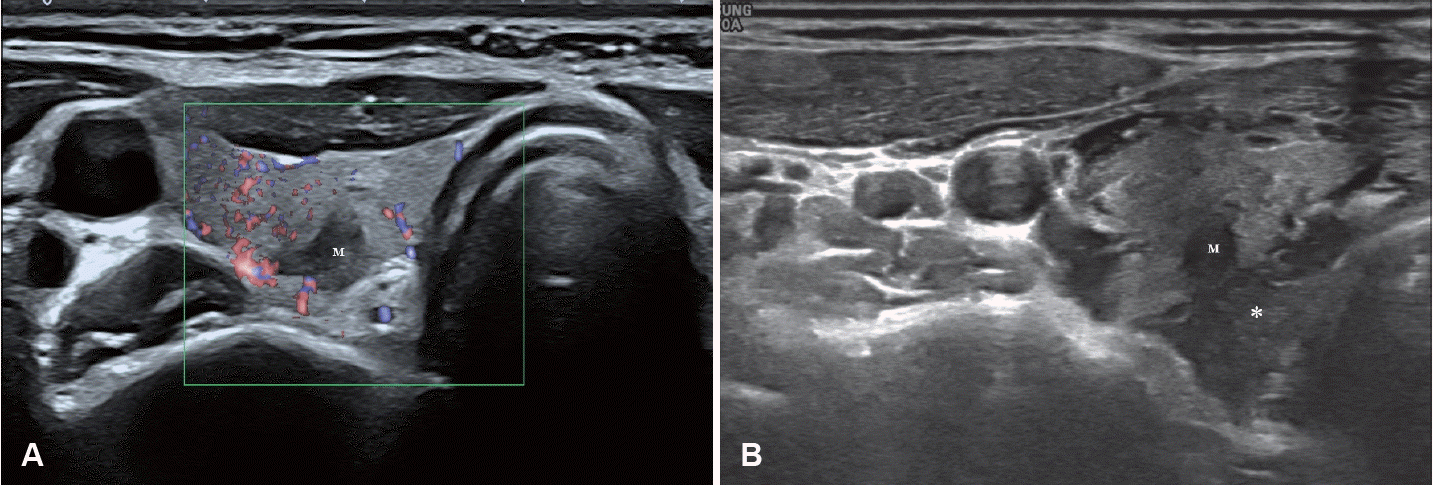
Anterolateral hydrodissection. A: A mass (M) is identified in the right thyroid gland. B: Anterolateral hydrodissection is performed by instilling a cold 5% dextrose solution (asterisk) to separate the right thyroid gland from the carotid artery (C). Arrows indicate 25-gauge needle inserted for injection.
Lateral, longitudinal, and oblique approaches
While the trans-isthmic approach is the basic approach for performing RFA, it is just basic method aimed at avoiding risk areas such as the recurrent laryngeal nerve. For further efficient RFA, it is necessary to appropriately utilize lateral, longitudinal (Fig. 6A), or oblique (Fig. 6B) approaches depending on the location and characteristics of the nodule. Because real-time ultrasound provides sufficient awareness of risk areas and hydrodissection can be used to avoid vital structures for added safety, the use of a lateral, longitudinal, or oblique approach is not absolutely riskier compared to the conventional trans-isthmic approach.

Longitudinal and oblique approaches for radiofrequency ablation. A caudocranial insertion of the electrode (arrows) parallel to the long axis of the thyroid gland (A), or an oblique insertion (B), can also be used safely as long as the entire length of the electrode is monitored on real-time ultrasound. The trachea is not depicted in the current images as they are obtained from longitudinal (A) and oblique views (B). C, carotid artery.
Procedure Time and Energy
The ablation usually starts at 30-50 watts, and if a high-echoic zone does not form around the electrode needle within 5-10 seconds, the procedure is continued by increasing the power by 10 watts, using a maximum output of 80-120 watts. For successful outcomes in benign thyroid nodules, a total energy of 15-50 kJ is generally reported to be necessary [16,21,22]. However, depending on the size or characteristics of the lesion, blood flow distribution, and thyroid atrophic/hyperplastic conditions (chronic thyroiditis/Graves’ disease), a good volume reduction effect may be achieved with less energy, or more energy may be required without achieving the desired effect. Pain may indicate heating of the thyroid capsule or thermal diffusion outside the thyroid, in which case the treatment should be temporarily halted, and the position of the electrode should be adjusted or additional local anesthetic should be used.
Post-Procedure Management
In the early post-procedure period, ice packs can help reduce local swelling and discomfort. Although specific antibiotic recommendations post-RFA are lacking, the author prefers to use anti-inflammatory and antibiotic medications to prevent infection and manage pain. Immediate evaluation is needed for severe pain, localized swelling, erythema, fever, voice changes, dysphagia, or respiratory difficulty. Voice function is subjectively assessed and objectively confirmed through laryngoscopy.
At the author’s institution, RFA is performed in a day surgery setting, if no complications arise. Follow-up occurs 1 month post-procedure, then at regular intervals (3 months, 6 months, 1 year) to monitor clinical, imaging, and biochemical responses. Symptoms and cosmetic scores are documented using validated scales. Additional RFA sessions are considered if symptoms or hyperthyroidism persist or if the VRR is less than 50% at 3-6 months post-procedure.
Personal Experience with RFA
From January 2022 to June 2024, a total of 73 cases of RFA were performed, comprising 64 cases of benign thyroid nodules, 6 cases of recurrent/metastatic thyroid cancer, and 3 cases of primary papillary thyroid microcarcinoma. The treatment success rate (VRR ≥70%) was 89% (65/73). Eight patients did not achieve a treatment success after the first procedure, or continued to experience subjective discomfort, leading to additional RFA sessions. Two patients showed no response to RFA: one with diffuse goiter due to Graves’ disease showed no reduction in thyroid size after three RFA sessions, and another with a large thyroid mass (8.2×5.9×10.3 cm) in the left thyroid showed a VRR of less than 20% even after two RFA sessions. There were no major complications requiring intervention. One case of transient vocal cord paralysis was observed, possibly caused by the spread of injected lidocaine around the carotid space rather than by thermal injury from the RFA.
Discussion
RFA for thyroid lesions offers several advantages when performed by head and neck surgeons who are primarily responsible for such diseases [23,24]. First, head and neck surgeons are highly familiar with surgical anatomy and have extensive clinical experience with anatomical features and the relationships between surrounding structures. This expertise allows for a better understanding of the patient’s anatomical structures and potential injury outcomes, facilitating safer and more aggressive procedures. Second, although RFA is generally a very safe procedure, rare but serious complications such as bleeding, airway compression, recurrent laryngeal nerve paralysis, and tumor rupture can occur. When such situations arise, head and neck surgeons are well-prepared to respond immediately and appropriately. Third, while RFA is an effective treatment for benign thyroid nodules, achieving a VRR of 60%-80%, 10%-30% of patients may experience a VRR of less than 50% or require additional surgery due to regrowth of the nodule [6,7,13,15]. In these cases, head and neck surgeons can make more informed and integrated decisions regarding the necessity and method of additional surgery following RFA, and better manage tissue changes caused by RFA during surgery.
Even if head and neck surgeons do not directly perform RFA, a thorough understanding of its indications, techniques, efficacy, and complications can significantly broaden the range of treatment options available to patients and clinicians. For instance, similar to the approach of reducing tumor size and extent with neoadjuvant chemotherapy before surgery or radiation in head and neck squamous cell carcinoma, RFA can be used to reduce the size of large benign thyroid nodules prior to surgery, thereby decreasing the extent of surgical resection and associated complications. Additionally, RFA is a viable treatment option for recurrent thyroid cancer in previously operated neck areas, offering patients and surgeons an alternative to repeated surgery with the potential for favorable long-term outcomes avoiding surgical burdens [25].
RFA is an important procedure that has brought a paradigm shift in the treatment of thyroid lesions. While it is currently primarily used for benign thyroid nodules, it is expected to become a mainstay treatment for both recurrent and primary thyroid cancer in the future. Therefore, it is essential for head and neck surgeons, who have traditionally provided surgery-based treatments, to integrate RFA into their practice. This requires a comprehensive understanding of the appropriate application of RFA, its outcomes, and continued efforts to advance the technique.
Acknowledgements
None
Notes
Author Contribution
Conceptualization: Dongbin Ahn. Data curation: Hyowon Ahn. Investigation: Dongbin Ahn. Methodology: Soobi Ham. Project administration: Soobi Ham. Resources: Dongbin Ahn, Soobi Ham. Software: Soobi Ham. Validation: Hyowon Ahn. Visualization: Dongbin Ahn. Writing—original draft: Dongbin Ahn, Hyowon Ahn. Writing—review & editing: Dongbin Ahn.