소아 폐쇄성수면무호흡증: 임상 증상 및 합병증
Pediatric Obstructive Sleep Apnea: Clinical Manifestations and Consequences
Article information
Trans Abstract
Obstructive sleep apnea (OSA) significantly impacts the quality of life in children, affecting their physical and mental health. The pathophysiology involves a complex interaction between anatomical factors (such as enlarged tonsils, adenoids, and structural abnormalities) and obesity, along with neuromuscular compensations. Clinical manifestations include loud snoring, breathing pauses, restless sleep, unusual sleeping positions, and frequent awakenings. OSA can lead to enuresis, growth impairment, poor academic performance, and neurocognitive and behavioral difficulties. Long-term consequences are cardiovascular morbidity, metabolic dysfunction, and neurocognitive impairment. Many studies have explored these associations and the impact of adenotonsillectomy. This review provides an overview of clinical manifestations and consequences of OSA, aiming to enhance diagnosis and treatment strategies for pediatric OSA.
Introduction
Pediatric obstructive sleep apnea (OSA) is a sleep disorder characterized by repetitive collapse of the upper airway during sleep, resulting in partial or total obstruction of breathing. The prevalence of OSA is estimated to be between 1% and 5% of preschool and school-aged children [1,2]. OSA in children is reported to be similar in boys and girls and is more common in children who are overweight or obese, have neuromuscular disorders, or have craniofacial abnormalities [3,4]. OSA can have a significant impact on children’s quality of life, physical and mental condition.
The clinical manifestations of OSA in children typically involve disruptions in respiration during sleep such as loud or persistent snoring, pauses in breathing. In addition, pediatric OSA can lead to a variety of signs and symptoms, including restless sleep, frequent awakenings, unusual sleeping positions, paradoxical chest movement, enuresis, growth impairment, poor academic performance, neurocognitive and behavior difficulties [5]. If pediatric OSA remains undiagnosed and untreated, serious medical problems could occur.
The long-term consequences of OSA in children can be varied and serious. Pediatric OSA has been linked to an increased risk of cardiovascular morbidity (e.g., blood pressure [BP] dysregulation, endothelial dysfunction), metabolic dysfunction (e.g., insulin resistance, dyslipidemia), and neurocognitive impairment (e.g., attention-deficit/hyperactivity disorder [ADHD], neurocognitive deficit) [6-9.] Therefore, early diagnosis and treatment of OSA in children are essential to prevent these long-term consequences.
The purpose of this review is to provide a comprehensive overview of the clinical manifestations and consequences of OSA in children. This review will contribute to identifying clinical features including signs and symptoms of pediatric OSA, understanding potential complications of OSA in children, and developing effective diagnostic and treatment strategies for pediatric OSA.
Pathophysiology
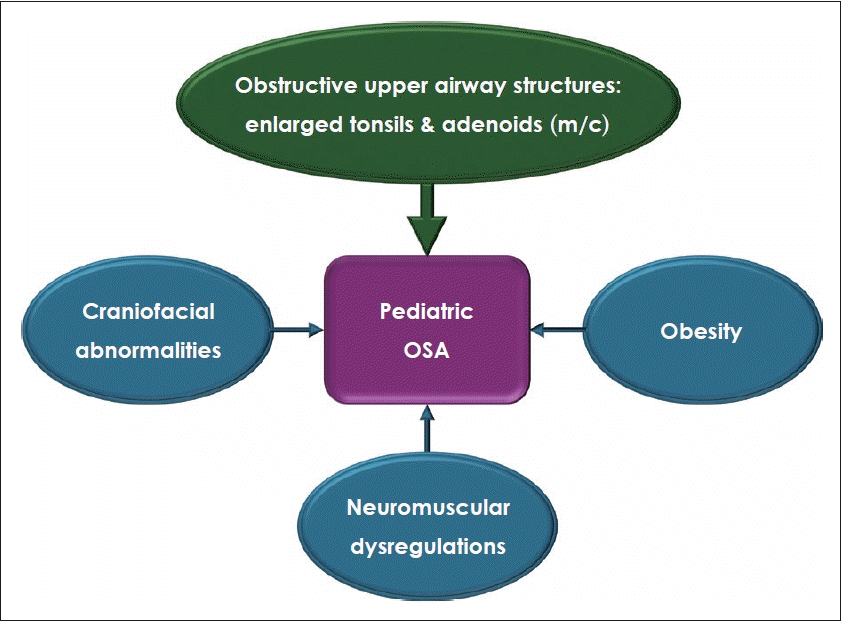
The pathophysiology of OSA in children is a complex interaction between upper airways prone to collapse, including anatomical factors or structural abnormalities and obesity, and neuromuscular compensations or regulations (Fig. 1). Anatomical factors are composed of a variety of upper airway structures, such as inferior turbinates, nasal septum, adenoids, soft palate, tonsils, and tongue [10]. The upper airway can be narrowed or even obstructed due to individual or multiple abnormalities in these upper airway structures.
Enlarged tonsils and adenoids are the most common cause of pediatric OSA [5,11]. The peak age for tonsil and adenoid hypertrophy is known to occur between the ages of 3 and 7. Enlarged tonsils and adenoids are well-known to cause higher nasal airway resistance and reduced pharyngeal volume, leading to nasal obstruction and subsequent mouth breathing. This condition can result in alterations in facial growth and dental arch morphology, including vertical growth, mandibular retrognathia, and malocclusion [12-14]. The impact of enlarged tonsils and adenoids on the upper airway and the development of OSA has been demonstrated through changes observed following adenotonsillectomy and long-term follow-up studies [12,15]. This highlights the need for careful monitoring and timely surgical intervention to manage these conditions effectively.
In addition, upper airway collapse can occur caused by various factors, such as obesity and craniofacial anomalies. Obesity increases the risk of OSA by narrowing the upper airway through increased tissue mass, reduced muscle tone, and changes in functional mechanisms modulating upper airway patency [16]. Obesity-related pediatric OSA may be more prevalent in older age groups compared to those where tonsil and adenoid enlargement is the predominant cause [17]. Craniofacial abnormalities are structural defects in the face and skull that can result from genetics, birth defects, or environmental exposures. Common skeletal abnormalities linked to OSA include micrognathia and midface hypoplasia, along with associated congenital disorders such as Down syndrome, Pierre Robin sequence, and Treacher Collins syndrome [18-20]. Neuromuscular compensations or regulations is related to arousal, respiratory effort, neuromuscular reflexes in the upper airway, and neuromuscular disorders such as Duchenne muscular dystrophy and spinal muscular atrophy [21]. In children with OSA, a variety of factors may cause these neuromuscular compensations or regulations to be insufficient to prevent upper airway obstruction. This can induce repetitive episodes of respiratory events including snoring, respiratory effort-related arousal, hypopnea, and apnea.
In general, similar to adult OSA, pediatric OSA also shows the interruption of airflow despite respiratory efforts, arousal or oxygen desaturation. However, occasionally in children, OSA may manifest as obstructive hypoventilation accompanied by hypercapnia, snoring or paradoxical breathing [22]. Therefore, these features are included in the diagnostic criteria for pediatric OSA. Upper airway collapses caused by various factors described above causes repeated hypoxia, hypercapnia, hyperactivation of the sympathetic nervous system, sleep fragmentation, and exaggerated negative intrathoracic pressure. As a result, the persistence of these pathophysiological phenomena leads to the occurrence of various symptoms and critical consequences.
Clinical Manifestations
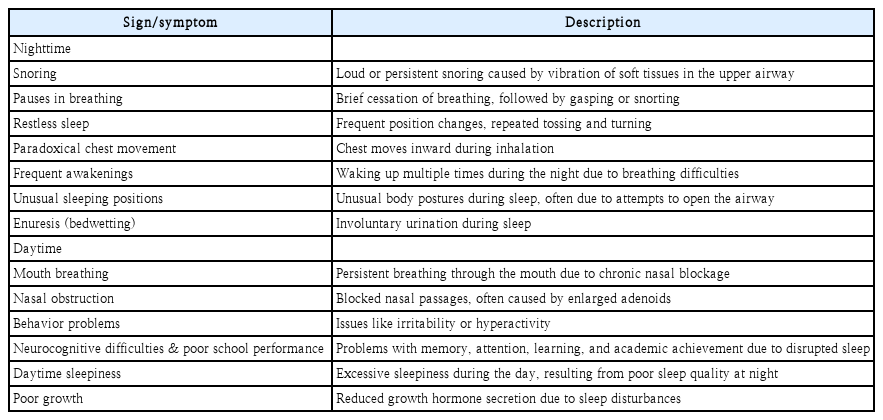
OSA in children can result in diverse clinical manifestations, including both nighttime and daytime symptoms. There are many signs and symptoms during nighttime in children with OSA such as snoring, pauses in breathing, restless sleep, paradoxical chest movement, frequent awakenings, unusual sleeping positions, and enuresis [23]. Signs and symptoms during daytime in pediatric OSA include mouth breathing, nasal obstruction, behavior problems, neurocognitive difficulties, poor school performance, daytime sleepiness, and poor growth (Table 1) [24,25].
Snoring ensues from the vibration of soft tissues in the upper airway, occurring as the child contends with breathing difficulties. Loud or persistent snoring is a defining feature of pediatric OSA [26]. However, it cannot be presumed that every child who snores has OSA. Nevertheless, snoring, especially loud or persistent, requires heightened attention because it indicates a negative impact on normal respiration.
Pauses in breathing is a meaningful sign and symptom of pediatric OSA, it can occur numerous times per night. Interruptions in respiration can persist for several seconds or even longer. Parents or caregivers may observe instances where the child ceases breathing during sleep, followed by an abrupt gasp or snort as they resume breathing [27].
Restless sleep can be recognized by frequent changes in sleep positions, repeated tossing and turning, or the display of indications of discomfort during sleep [28]. Children with OSA who have restless sleep often experience disruptions in their sleep architecture, leading to an overall reduction in sleep quality. These restless sleep patterns may give rise to various additional symptoms in pediatric OSA.
Paradoxical chest movement refers to an atypical respiratory pattern where the chest moves in an inward manner during inhalation instead of the expected outward expansion. This paradoxical movement indicates heightened respiratory effort in response to upper airway obstruction and is frequently observed in children with OSA [29]. These abnormal chest dynamics add to the respiratory distress encountered by pediatric individuals affected during their sleep.
Enuresis in children, commonly known as bedwetting, is a condition characterized by the involuntary release of urine during sleep. It may be considered normal in younger children, but this condition can be associated with disruptions in respiratory disturbances and sleep patterns posed by OSA. It is recognized that enuresis may be more prevalent among children experiencing OSA, a condition marked by breathing interruptions during sleep [30].
Consequences
The long-term consequences of pediatric OSA can be broadly classified into three morbidities.
Cardiovascular morbidity
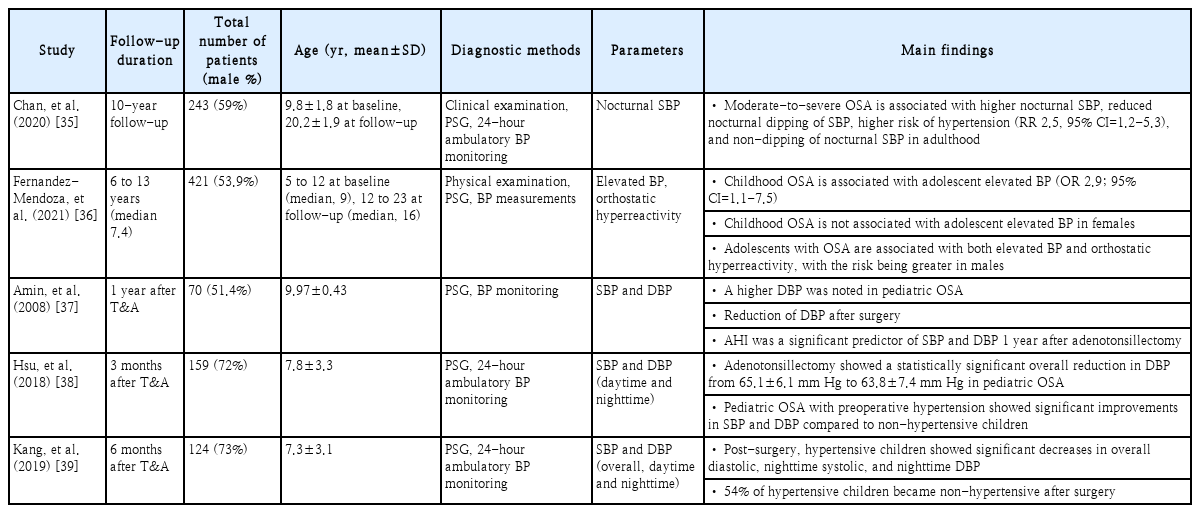
The autonomic nervous system (ANS), comprising the parasympathetic and sympathetic divisions, maintains homeostasis and highly regulates the cardiovascular system, resulting in different outcomes based on the balance of these opposing systems. The ANS, through the baroreflex, tightly regulates BP by controlling cardiac output and vascular resistance, with parasympathetic activation rapidly slowing heart rate and sympathetic activation slowly increasing heart rate [31]. Recurrent hypoxemia and hypercapnia in pediatric OSA activate the sympathetic nervous system and stimulate peripheral chemoreceptors, causing systemic vasoconstriction and elevated systemic BP during apnea episodes [32]. The association between pediatric OSA and elevated BP has been established in several previous cross-sectional studies. One study found that children with OSA had significantly higher BP during both sleep and wakefulness compared to healthy controls, with children having moderate to severe OSA showing significantly higher odds of nocturnal systolic (odds ratio [OR] 3.9, 95% confidence interval [CI]=1.4-10.5) and diastolic (OR 3.3, 95% CI=1.4-8.1) hypertension [33]. Another study found that in elementary school children, the apnea-hypopnea index (AHI) is associated with elevated systolic blood pressure (SBP), showing increases of 2.9 mm Hg for AHI ≥1, 7.1 mm Hg for AHI ≥3, and 12.9 mm Hg for AHI ≥5 [34]. Follow-up cohort studies have investigated the impact of pediatric OSA and the effects of adenotonsillectomy, a key treatment, on BP (Table 2). The main findings indicate that pediatric OSA increases SBP or diastolic BP, and treatment can reduce some BP indicators.
Intermittent hypoxia is acknowledged as a key factor in the development of comorbidities associated with OSA, with mechanisms such as reactive oxygen species overproduction and cellular damage leading to endothelial dysfunction [40]. The endothelium is a crucial cell layer in the vascular system that plays a significant role in regulating immune and inflammatory responses, coagulability, and BP [6]. A number of studies have confirmed the occurrence of endothelial dysfunction through the expression of components such as tumor necrosis factor-α, pro-inflammatory cytokines (IL-6, IL-8), monocyte chemoattractant protein-1, vascular cell adhesion molecule-1, and angiotensin II induced by intermittent hypoxia [41-45]. One of the studies conducted in children, measuring flow-mediated dilation (FMD) using ultrasound to assess vascular reactivity after ischemia, demonstrated that children with OSA showed impaired endothelial function compared to controls [46]. Specifically, higher AHI was associated with worse FMD values, indicative of more severe endothelial dysfunction. Another study found that compared to healthy controls, children with OSA had higher levels of soluble NADPH oxidase 2-derived peptide (sNOX2-dp), a marker of oxidative stress, and lower FMD, with adenotonsillectomy significantly reducing these oxidative stress markers and improving FMD, suggesting NOX2-derived oxidative stress involvement in endothelial dysfunction in pediatric OSA [47].
Studies have examined the association between echocardiographic findings and cardiac dysfunction in pediatric OSA. One study measured differences in children with OSA and primary snoring, finding an at least 11-fold increase in the risk for left ventricular (LV) hypertrophy in the OSA group, and suggesting that LV hypertrophy in pediatric OSA may develop independently of persistent elevated BP, secondary to episodic hypoxia [48]. Another study of 373 children found that children with OSA exhibited decreased diastolic function, reduced systolic function, and an elevated LV mass index at baseline when compared to the control group, with diastolic function worsening with increasing OSA severity [49]. In that study, adenotonsillectomy significantly improved diastolic function in pediatric OSA, but there were no significant changes in LV mass index. A study using Doppler imaging found that the pediatric OSA group had mean pulmonary artery pressure, higher pulmonary artery systolic pressure, pulmonary vascular resistance, LV mass index, and right ventricular diastolic diameter compared to healthy controls, with these findings being reversible after adenotonsillectomy [50].
A study using the National Health Insurance Research Database compared 6535 children and adolescents with OSA to a control group [51]. After 15 years, the OSA group had a 2.05 times higher incidence of major adverse cardiovascular events, including coronary artery disease, acute myocardial infarctions, peripheral artery disease, and acute stroke. Notably, no cardiovascular events were found in those who received continuous airway positive pressure treatment or pharyngeal surgery. Elevated BP, abnormal echocardiographic findings, vascular abnormalities, and various blood tests indicative of cardiovascular risk have been documented in numerous studies on pediatric OSA. Clinically, these abnormalities are likely to improve with adenotonsillectomy. Further prospective studies are needed to determine the impact of these improvements on cardiovascular morbidity in adulthood.
Metabolic dysfunction
Sleep is known to play a crucial role in metabolic processes. In OSA, respiratory events, decreased oxygen saturation (SaO2), sleep fragmentation, and reduced sleep quality are presumed to influence various metabolic hormones and contribute to metabolic dysfunction [8,52]. Especially, the relationship between pediatric OSA and growth has been highlighted in several studies. One such study found that the prevalence of growth failure was significantly higher in children with OSA, with height-for-age ≤3rd percentile observed in 7.56% of children with OSA compared to 2.91% of healthy children, and weight-for-age ≤5th percentile in 9.30% compared to 2.33%, respectively [53]. Significant improvements in growth parameters were observed following adenotonsillectomy. Another study of infants who underwent adenotonsillectomy showed a significant improvement in weight gain velocity in those diagnosed with failure to thrive (FTT), highlighting the importance of considering OSA in the differential diagnosis of FTT [54]. Growth hormone is thought to be primarily secreted during deep sleep, and reports indicating that children with growth hormone deficiency exhibit reduced total sleep time and sleep efficiency suggest a potential correlation between sleep structure and growth hormone [55,56].
Obesity has been shown to cause various metabolic dysfunctions, including hypercholesterolemia, hyperglycemia, dyslipidemia, and insulin resistance, significantly increasing the risk of cardiovascular disease [57,58]. There are differing views on whether obesity and OSA independently contribute to metabolic dysfunction or if obesity alone is responsible. To clarify this, the results of studies controlling for body mass index (BMI) are essential. In a study on obese children found that patients with OSA had significantly higher fasting insulin, blood glucose, and homeostasis model assessment (HOMA) levels compared to those without OSA, and analyzed that fasting insulin and HOMA predict severe OSA independent of age, gender, and BMI z-score [59]. Another study on children who underwent adenotonsillectomy due to OSA, 6-12 months after the surgery, no changes in fasting glucose, insulin, or insulin/glucose ratio were observed in nonobese children; however, in obese children, significant improvements in fasting insulin and insulin/glucose levels were noted, even in the absence of any BMI changes [60]. On the other hand, a study reported that after controlling for BMI, the association between AHI and insulin resistance disappeared [61]. However, a meta-analysis of eight studies on pediatric OSA and insulin resistance confirmed that insulin resistance, measured by HOMA, was higher in the OSA group (g=0.78, 95% CI=0.25-1.31) [62].
A study involving 191 Korean children and adolescents found that high-density lipoprotein cholesterol (HDL-C) levels were significantly lower in those with OSA compared to those without, regardless of obesity, and differed significantly between OSA severity groups after adjusting for BMI, indicating that OSA may be an independent risk factor for dyslipidemia [63]. Another study found no significant correlation between AHI and serum lipids, and suggested that in pediatric OSA, dyslipidemia is primarily determined by relative BMI rather than AHI [64]. In a meta-analysis examining the correlation between pediatric OSA and dyslipidemia, results showed that triglyceride levels were not statistically significant in OSA patients compared to controls, total cholesterol levels were higher in OSA patients with a small effect (g=0.28, 95% CI=0.01-0.55), and HDL-C levels were significantly lower in OSA patients (g=0.37, 95% CI=0.05-0.70) [62].
Although there is no unified definition for metabolic syndrome (MetS) in children, the International Diabetes Federation has provided specific criteria for those aged 10 to under 16, which include obesity defined as a waist circumference at or above the 90th percentile, triglycerides ≥150 mg/dL, HDL-C <40 mg/dL, SBP ≥130 mm Hg or DBP ≥85 mm Hg, and glucose ≥100 mg/dL or known type 2 diabetes mellitus, while recommending the use of adult criteria for those older than 16 [65]. Verhulst, et al. [66] found that in a study of 104 overweight and obese children and adolescents, mean SaO2 and SaO2 nadir were independent and significant predictors of MetS, with significant associations between SaO2 nadir and HDL-C, mean SaO2 and both glucose and triglyceride levels, and the percentage of total sleep time with SaO2 >95% and cholesterol levels, supporting the hypothesis that OSA interacts with metabolic dysfunction independently of body fat distribution in children and adolescents who are overweight and obese. In a study of 270 adolescents aged 13 to 16 years, it was found that those with OSA have significantly higher odds (6.49 times) of having MetS compared to those without OSA [67]. After adjusting for BMI, OSA was still associated with elevated SBP and DBP, low-density lipoprotein cholesterol, and fasting insulin levels. In children and adolescents, it is important to better recognize the significance of early detection and subsequent lifestyle adjustments to prevent the progression to MetS in the future, and to pay close attention to its association with OSA.
Neurocognitive impairment
Several cross-sectional studies have investigated the relationship between pediatric OSA and cognitive function. In a study of children aged 7-12, cognitive assessment using psychological tests, including the Wechsler Abbreviated Scale of Intelligence and others, revealed that children with OSA showed reduced general intellectual ability regardless of severity, along with higher impairment rates in both executive and academic functioning [68]. Meanwhile, another study on preschool children showed similar cognitive assessment results between primary snoring and various severity of OSA groups, suggesting the absence of notable cognitive impairment in this age group, despite poorer behavior being observed [69].
The relationship between cognitive function and pediatric OSA has been confirmed in several studies by comparing pre- and post-adenotonsillectomy outcomes. A representative assessment tool for evaluating cognitive function in children is the neuropsychological developmental assessment (NEPSY), which focuses on areas such as attention, language, sensorimotor functions, visuospatial processing, memory, learning, and social perception [70]. In a randomized controlled trial involving children aged 5 to 9 with OSA, with 194 in the adenotonsillectomy group and 203 in the watchful-waiting group, early adenotonsillectomy did not significantly improve NEPSY scores compared to watchful waiting, but it resulted in significant improvements in quality-of-life, behavioral, and polysomnographic measures [71]. A meta-analysis comparing pre- and post-adenotonsillectomy NEPSY scores in 375 children showed a significant increase from a mean±standard deviation of 101.5±14.7 to 108.8±13.4 [72]. Also, the study demonstrated a statistically significant increase in a comparison of intelligence quotient of 254 children from a mean±standard deviation of 97.1±13.8 to 100.7±11.1, but pointed out that the results were based on only three studies including preschool-aged children. In another meta-analysis evaluating the effects of adenotonsillectomy by age, it was found that older children (>7 years) showed greater improvements in neurocognitive and behavioral performance within 12 months post-surgery compared to pre-school aged children (≤7 years), with the duration of follow-up significantly influencing the effect size of these improvements, suggesting that the improvements were more related to the length of follow-up than the age at surgery [73].
In pediatric OSA, behavioral problems similar to those observed in ADHD, including social problems, thought problems, attention problems, rule-breaking behavior, and aggressive behavior, can be assessed through caregiver-reported questionnaire scores. Several studies have identified the presence of behavioral problems in children with OSA, and it has been observed that adenotonsillectomy can significantly improve these problems [71,74,75]. A representative randomized trial involving children aged 5-9 with OSA demonstrated significant improvement in total problems, internalizing behaviors, somatic symptoms, and thought disturbances after early adenotonsillectomy compared to a watchful waiting approach with supportive care, even after excluding sleep-specific items in the analysis [76].
There is still much to be understood about the mechanisms leading to neurocognitive dysfunction in pediatric OSA. Beebe and Gozal [77] suggested that sleep disruption and intermittent hypoxia, along with hypercapnia, might disrupt restorative sleep features and cellular or chemical homeostasis, potentially leading to prefrontal cortical dysfunction. This dysfunction of the prefrontal cortex could subsequently cause impairments in the cognitive executive system, possibly resulting in behavioral deficits such as problems with mental manipulation of information, poor planning, disorganization, and difficulties in maintaining attention and motivation.
Conclusion
Pediatric OSA is associated with three major consequences: cardiovascular morbidity, metabolic dysfunction, and neurocognitive impairment. Many clinical studies have investigated these associations and assessed the effects of adenotonsillectomy on these conditions. The clinical significance of pediatric OSA lies in the potential for early intervention, including surgical treatment, to prevent these adverse outcomes. Given that pediatric OSA often presents with different clinical manifestations compared to adult OSA, recognizing these differences is essential for ensuring timely diagnosis and effective management.
Acknowledgements
This study was supported by the Soonchunhyang University Research Fund.
Notes
Author contributions
Conceptualization: Ji Ho Choi. Investigation: Ji Ho Choi, Ji-Sun Kim. Supervision: Ji Ho Choi. Writing—original draft: Ji-Sun Kim, Ji Ho Choi. Writing—review & editing: Ji Ho Choi, Ji-Sun Kim.