비용종을 동반한 만성비부비동염 환자에서 두필루맙 치료의 효용성 및 안정성: 1개월 간격 투여에 대한 후향적 분석연구
Efficacy and Safety of Dupilumab for Chronic Rhinosinusitis With Nasal Polyps: A Retrospective Study of Every Month Injection
Article information
Trans Abstract
Background and Objectives
Dupilumab is approved for the treatment of uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP) and has been shown to be relatively safe and effective in randomized controlled trials. As such, real-world effectiveness and safety data should be obtained.
Subjects and Method
We performed a retrospective review of patients with CRSwNP who received monthly treatment of dupilumab between January 2022 and June 2023. Reviewed for the study were the following: demographic data, comorbidities, the visual analogue scale (VAS) for nasal obstruction and sense of smell, identification scores of the Korean version Sniffin’ stikc II (KVSS II) test, nasal polyp score (NPS), and serum eosinophil count. Statistical analyses were performed for each clinical variable.
Results
A total of 76 patients (49 male, 27 female) were included in this study. The VAS scores decreased from 7.17 at the baseline to 4.51 at month 6; the KVSS II identification scores increased from 6.71 to 8.47, and the NPS decreased from 3.82 to 0.44. The sino-nasal outcome test-22 scores decreased from 36.29 at the baseline to 8.22 at month 6. The correlations between all clinical variables were statistically significant.
Conclusion
Monthly treatment of dupilumab is effective and safe for patients with CRSwNP. Further research is required to determine the predictive parameters for treatment responses and adverse events.
Introduction
Chronic rhinosinusitis (CRS) is a common inflammatory disease affecting approximately 5%-12% of adults worldwide and approximately 8.4% of the Korean population [1,2]. If nasal polyps are present, both rhinosinusitis and polyps show higher recurrence rates of approximately 20%-60% after endoscopic sinus surgery [3]. Thus, patients have undergone revision endoscopic sinus surgery or long-term corticosteroid treatment, which leads to an increased cost burden and lowers the quality of life.
Dupilumab is a human monoclonal IgG4 antibody that binds to the interleukin-4 (IL-4) receptor α subunit to inhibit IL-4 and IL-13, which play a key role in type 2 inflammation [4]. At the molecular level, inhibition of IL-4 and IL-13 reduces type 2 biomarkers (e.g., eotaxin-2, total IgE, and pulmonary and activation-regulated chemokine).
In 2019, the US Food and Drug Administration approved dupilumab for the treatment of uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP). The efficacy and safety of dupilumab in patients with CRSwNP have been reported in two large multicenter randomized controlled trials [5]. In these trials, patients got 300 mg of dupilumab every 2 weeks during study periods.
However, to the best of our knowledge, reports based on real-world experiences remain insufficient in Korea. Furthermore, most of patients visiting our center took dupilumab every month because they couldn’t afford cost of medication. Thus, we wanted to figure out efficacy and safety of dupilumab every month for patients with uncontrolled CRSwNP.
Subjects and Methods
Study design
We performed a retrospective chart review of patients who received dupilumab every month for the treatment of CRSwNP at the Department of Otorhinolaryngology-Head and Neck Surgery of Kosin University Gospel Hospital between January 2022 and June 2023. The study procedure was exempted from human subject research by the Institutional Review Board (IRB) of Kosin University Gospel Hospital (IRB number KUGH 2023-04-033).
Inclusion and exclusion criteria
Patients who were diagnosed to uncontrolled CRSwNP and administrated 300 mg of Dupixent® (dupilumab; Paris, France) subcutaneously every month were included. Term ‘uncontrolled’ was defined to whose nasal symptoms such as rhinorrhea, nasal congestion and hyposmia or anosmia continued over 1 year while use of systemic corticosteroids more than two courses or use of low dose corticosteroids over 3 months.
Patients whose age was under 18 years at start of dupilumab treatment were excluded. Patients who received dupilumab less than 3 times or who had not received injections over a 2 months span were excluded for proper comparison. Patients who received systemic or local corticosteroids for other diseases, and those who received other monoclonal antibody or immune therapies during dupilumab administration were excluded.
Data collection
Demographic information, including age and sex, was collected. The presence of underlying diseases, including other type 2 inflammatory diseases (e.g., asthma and atopic dermatitis), was recorded.
Clinical variables—including the visual analogue scale (VAS—ranging from 0 to 10) for nasal symptoms that include nasal obstructions, rhinorrhea, and hyposmia; changes in the endoscopic nasal polyp score (NPS—ranging from 0 to 8, with higher scores indicating a higher polyp burden); and the identification score of the Korean version of the Sniffin’ stick II (KVSS II) test involving six odors familiar to Koreans (patients smells each of 16 odors and choose one out of four odors items even though they do not know the correct one. And the number of correct answers is scored [6].)—were collected before each injection for clinical outcomes. Serum eosinophil counts (cells/μL) and tissue eosinophil counts of biopsy specimen of nasal polyps (cells/HPF) were collected from patients who underwent sinus surgery.
European Position Paper on Rhinosinusitis and Nasal Polyps/European Forum for Research and Education in Allergy and Airway Diseases 2023 (EPOS/EUPOREA 2023) guidelines suggested indications for biological treatment in CRSwNP as five criteria and cut-off points (Table 1) [7]. We analyzed whether patients met each criteria retrospectively. And the EPOS/EUPOREA 2023 guidelines suggested for evaluating the response to treatment after 6 months and 1 year based on five criteria: reduced nasal polyp size, reduced need for systemic oral corticosteroids, improved quality of life, improved sense of smell, and reduced impact of comorbidities. Based on these criteria, we evaluated the response of treatment every 3 months.

Criteria and cut-off points of indication for biological treatments in chronic rhinosinusitis with nasal polyps of EPOS/EUFOREA 2023 guideline
All clinical variables except tissue eosinophil counts (measured once at the sinus surgery) and sino-nasal outcome test-22 (SNOT-22; measured at the beginning of the treatment and every 6 months) were collected at the beginning of the treatment, at month 1, and every 3 months thereafter.
Statistical analysis
Descriptive statistical analysis, Wilcoxon signed-rank tests, and Mann-Whitney U tests were performed using SPSS (version 25.0; IBM Corp., Armonk, NY, USA). To assess the risk factors associated with the development of adverse events, qualitative variables were compared using the chi-squared test (or Fisher’s exact test when necessary). Student’s t-test was used to compare the distribution of quantitative data (or the Mann-Whitney U test when the distribution departed from normality or when homoscedasticity was rejected). A p-value under 0.05 is considered to indicate statistical significance.
Results
Demographics
A total of 81 patients were enrolled. Five patients were excluded; two patients whose age were under 18 at start of the dupilumab administration and three patients who were administrated dupilumab under 3 times during study period. Thus, 76 patients (49 of male and 27 of female) were finally included in this study (Fig. 1). All of included patients were administrated 300 mg dupilumab subcutaneously 1-month interval during the study period.
The mean age at the start of therapy was 48.2 years (with a range of 19-83). A total of 72 of the 76 participating patients had previously undergone one or more endoscopic sinus surgeries. Nine patients had coexisting type-2 inflammatory disease, all of whom had asthma. None of the patients had other type 2 inflammatory diseases, such as atopic dermatitis. Patients got an average of 4.06 injections (with a range of 2-12).
Baseline characteristics
The baseline clinical variables were collected during the first clinic visit. We subsequently analyzed the changes in clinical variables during a follow-up visit. Clinical variables, except serum eosinophil counts, improved significantly. The mean VAS score for nasal symptoms was 7.17, with 50 patients (65.8%) presenting a score of 7 or higher. The mean SNOT-22 score was 36.29. The mean NPS was 4.06 (with a range of 2-7) with 48 patients (63.1%) falling in the 4-8 range and 23 patients (30.3%) falling within the 1-3 range. The mean Identification score of the KVSS II was 6.71 (with a range of 0-13). The mean serum eosinophil count was 363 cells/μL. Seventeen patients had an eosinophil count higher than 500 cells/μL of serum (hypereosinophilia); however, no patient presented with an eosinophil count higher than 1500 cells/μL (Table 2).
Clinical variables and outcomes
Mean identification score of KVSS II was increased from 6.71 at baseline to 8.47 at month 1 (p<0.001), 8.97 at month 3 (p<0.001), and 9.69 at month 6 (p<0.001). The increase in score was most prominent after the first injection (Fig. 2A).

Box plots for each clinical variable. Blue “X” indicates the statistical mean. Shown are the baseline and visits at months 1, 3, 6 (months 1 and 6 for SNOT-22). A: Identification score of KVSS II. B: VAS for nasal symptoms. C: Nasal polyp score. D: Serum eosinophil count. E: SNOT-22 score. SNOT, sino-nasal outcome test; KVSS, Korean version Sniffin’ stikc; VAS, visual analogue scale; ENDO, nasal polyp score; EOS, serum eosinophil count.
The mean VAS scores for nasal symptoms decreased from 7.17 at baseline to 4.51 at month 1 (p<0.001), 3.77 at month 3 (p<0.001), and 2.64 at month 6 (p<0.001). Similar to the KVSS II identification score, the change after the first injection was the most prominent. At month 6, four patients presented with VAS scores higher than four. None of the patients required systemic corticosteroids after the first dupilumab injection (Fig. 2B).
The mean NPS decreased from 3.82 at baseline to 2.02 at month 1 (p<0.001), 1.34 at month 3 (p<0.001), and 0.44 at month 6 (p<0.001). None of the patients had an NPS >3 at month 6 (Fig. 2C).
The serum eosinophil count decreased from 363 to 361 cells/μL at month 6—however, this is not statistically significant. Twelve of the 76 patients showed signs of hypereosinophilia at baseline. Three patients showed newly onset transient elevations of serum eosinophil at month 1. All eosinophil counts were under 1500 cells/μL (Fig. 2D).
The SNOT-22 score decreased from 36.29 at baseline to 8.22 at month 6 (p<0.001); in two of the patients, the SNOT-22 score at month 6 was higher than 40 (Fig. 2E).
Response to the treatment
Based on EPOS/EUPOREA 2023 guidelines, 34 patients showed good to excellent responses (met criteria 4 to 5), 33 patients showed poor to moderate response (met criteria 1 to 3), and one patient showed no response (met no criterion) at month 1. A total of 35 patients showed a good-excellent response and 26 patients showed a poor-moderate response at month 3. Lastly, 26 patients showed a good-excellent response and 10 patients showed a poor-moderate response at month 6 (Fig. 3). In detail, all nine of the patients with comorbid asthma showed a reduced impact had by the asthma. None of the patients required oral corticosteroids while being administered dupilumab.

Histogram of patients’ response to dupilumab treatment as per European Position Paper on Rhinosinusitis and Nasal Polyps/ European Forum for Research and Education in Allergy and Airway Diseases 2023 guidelines. Green shows the number of patients with a “good-excellent response,” orange for a “moderatepoor response” and red for “no response.”
During dupilumab administration, 7 of 76 patients (9.2%) needed additional medications to regulate CRSwNP. Four patients except for cases of adverse events took oral antihistamines within 1 week. Two patients took leukotriene receptor antagonist within 1 month. One patient took antihistamine to regulate rhinorrhea caused by COVID-19 infection. There was no patient who needed systemic or intra-nasal corticosteroids for their nasal symptoms except for cases of adverse events.
Safety
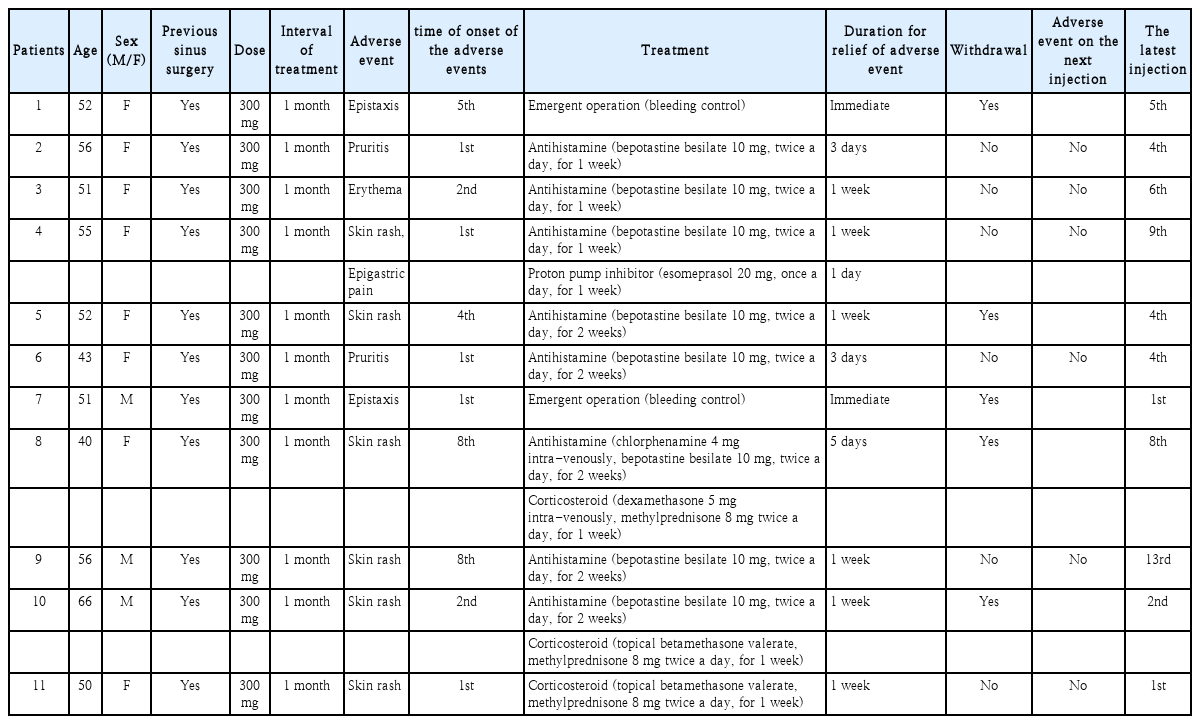
Eleven patients (14.5%) experienced 12 adverse events during the study period. The most frequent adverse event was a skin reaction other than at the injection site (9 of 12 adverse events), two cases of epistaxis, and one case of epigastric pain. No critical adverse events were observed. The injection site or ocular reactions, including conjunctivitis, were not recorded. None of the patients with hypereosinophilia showed organ symptoms such as fibrosis, thrombosis, erythema, and angioedema (Table 3).
“Skin reactions” included four cases of erythematous papules and patches, two cases of psoriatic rashes, two cases of pruritus, and one case of erythematous changes. All skin rashes regressed within 1 week of antihistamine and/or topical corticosteroids administration. Two patients with skin rashes discontinued dupilumab treatment at the physician’s discretion. No recurrence of skin rashes was observed in the patients who continued treatment. Two cases of epistaxis could have occurred postoperatively; however, in these cases, epistaxis occurred at least 1 month after sinus surgery. Intraoperative diffuse oozing and bleeding in both nasal cavities were observed in both cases, which differ from the general postoperative epistaxis.
Discussion
The treatment of choice for uncontrolled CRSwNP has varied with the development of biologics such as dupilumab. The efficacy and safety of dupilumab for CRSwNP have been reported in large multicenter randomized controlled trials (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52) [5]. In these trials, patients treated with dupilumab every 2 weeks for 24 (SINUS-24) and 52 weeks (SINUS-52) showed significant improvement in clinical outcomes, including nasal symptoms, nasal polyp size, sense of smell, and the need for systemic corticosteroids.
In our study, patients received dupilumab treatment at a relatively longer interval than that in the LIBERTY NP trials and other previous real-world studies. Patients received dupilumab injections every 2 weeks in most previous studies but every 4 weeks in this study [5,8-13]. This was due to the relatively higher price burden of dupilumab for patients under the Korean National Health Insurance system. Nevertheless, clinical variables improved significantly. The treatment resulted in rapid improvements in the NPS, nasal symptoms, olfactory performance, and the need for systemic corticosteroids.
Four patients did not get an endoscopic sinus surgery before the dupilumab administration. One patient did not fit systemic condition for the surgery and three patients refused the surgery and wanted to get the dupilumab treatment. At baseline, their clinical variables including KVSS II identification score, NPS, VAS, SNOT-22 score were similar to entire results. At month 6, all clinical variables got improved; KVSS identification score was 5.50, NPS was 0, VAS was 5.50 and SNOT-22 score was 12.00. Objective variables improved similar with entire results, but subjective variables were not improved as much as entire results. Because of small sample size, precise statistical analysis was not available. Patients who got the surgery before had relatively widened nasal cavity, thus they would be able to feel more comfortable after polyps were decreased than patients who did not get the surgery.
Consistent with previous real-world studies, clinical outcomes—including NPS, SNOT-22 score, and olfactory performance—showed statistically significant improvements. In previous studies, NPS decreased from 4.3-5.7 at baseline to 1.88-2.53, SNOT-22 decreased from 51.59-60.56 at baseline to 19.5-26.45, and SSIT-16 increased from 2.86-6.1 to 6.3-10.85, all at month 6 or 7. Detailed comparisons are described in Table 4.
In our study, serum eosinophil counts decreased during the treatment period, but the decrease was not statistically significant. A few patients showed a transient increase in eosinophil count; however, the overall average eosinophil count decreased. This is in contrast to previous real-world studies that showed a transient increase in the average eosinophil count [8,10]. Transient blood eosinophilia is thought to be caused by a decrease in eotaxin-3, which prevents eosinophils from migrating from the serum to the tissues [5]. Further investigation is needed to reveal the factors related to the eosinophil count in dupilumab-treated CRSwNP patients.
The profile of adverse events differed from those of previous studies. Except for temporary elevation of the serum eosinophil count, the frequent adverse events in previous studies included ocular reactions, injection site reactions, and arthralgia. In contrast, the occurrence of skin rashes, other than those at the injection site, was the most frequent adverse event observed in this study. There were no cases of arthralgia, ocular reactions, or injection-site reactions. No critical adverse events were observed (Table 5).
No defined factors are known to correlate with each adverse event. However, the injection interval could be one of the factors causing the different profiles of adverse events. Further studies are needed to determine whether the injection interval or race is one of the factors correlated with each adverse event.
Our study has several limitations. Data were collected retrospectively and, therefore, it was impossible to perform a placebo-controlled study. Additionally, there were missing values in the retrospectively collected data. Only the identification score of KVSS II was checked because the clinical system of our institute couldn’t afford the time needed for full examination. However, we could check the improvement of identification of smell test similar to other study using the identification test to check the olfaction [5,14,15].
Also, there were 35 patients who didn’t met EPOS/EUPOREA 2023 criteria. There were some reasons in selecting patients. First, we sent most of patients with asthma who needed corticosteroid to the department of allergology. Second, only 25 of 76 patients reported their SNOT-22 score over 40. Thus, there could be exist selection bias on our study. However, baseline characteristics of clinical variables were similar to previous studies. A prospective study would be needed.
Fourty of 76 (52.6%) patients didn’t took the dupilumab at month 6. Eleven of those patients started dupilumab treatment relatively late (when less than 6 months left until the end of the study period. Cessation of the treatment was mainly due to as follows: patient’s request and loss of followup. In addition, in the subgroup analysis, there were no significant differences in improvements in clinical variables between patients who continued treatment and those who did not. Thus, we could exclude the possibility that the 3 and 6-month data were biased to a certain extent.
In conclusion, our study suggests that dupilumab treatment every month, not every 2 weeks for uncontrolled CRSwNP results in rapid improvements in nasal polyposis, olfaction, and nasal symptoms. Dupilumab treatment is relatively safe with no critically adverse events observed. The most common adverse event was skin rash, which differs from the findings of previous studies. Long-term evaluations of the efficacy and safety of dupilumab and the persistence of treatment are needed.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Notes
Author Contribution
Conceptualization: Jooyeon Kim, Pooreum Kang. Data curation: Pooreum Kang, Donggyu Choi. Methodology: Jaehwan Kwon, Jooyeon Kim. Project administration: Jooyeon Kim. Resources: Jooyeon Kim, Jaehwan Kwon. Software: Donggyu Choi. Supervision: Jooyeon Kim. Visualization: Pooreum Kang, Donggyu Choi. Writing—original draft: Pooreum Kang. Writing—review & editing: Jooyeon Kim, Jaehwan Kwon.