 |
 |
AbstractBackground and Objectives The aim of this study was to confirm the effect of intralesional triamcinolone acetonide injections (TRIAM) to treat granulomatous tissue surrounding the tracheostomy stoma.
Subjects and Method We reviewed and documented the medical charts of 20 patients who were administered with TRIAM to treat granulomatous tissue surrounding the tracheostomy stoma from January 2018 to June 2019 were. The surface area of the granulomatous tissue was measured using Image J. The differences between the area of the granulomatous tissue after conventional treatment and after TRIAM on the same patient were compared.
Results A total of 20 patients consisting of 12 males and 8 females were included, with the patients’ average age being 60.0±14.3 years. The initial surface area of granulation tissue was 1.266±0.449 cm2, and 1.243±0.432 cm2 after conventional treatment, showing no statistically significant difference in the tissue area (p=0.143). The pre-injection surface area of granulation tissue was 1.243±0.432 cm2, and the area on the 7th day after the third injection was 0.477±0.217 cm2, showing a significant difference (p<0.001).
Conclusion Compared to the conventional treatment, the surface area of granulomatous tissue surrounding the tracheostomy stoma significantly decreased after being treating with TRIAM. This finding suggests the effectiveness of TRIAM as a treatment of granulomatous tissue surrounding the tracheostomy stoma without complications such as bleeding.
IntroductionTracheostomy was first described by Antonio Musa Brasavola in 1546 as a procedure that creates a tracheocutaneous opening to secure the airway in acute upper airway obstruction, prolonged intubation, or cases of difficult intubation and also effectively treat pulmonary diseases [1]. Complications of tracheostomy can occur immediately after the procedure and can also manifest as delayed complications [2,3]. The most common delayed complication is the formation of granulation tissue, which occurs in about 0.3%-80% of tracheostomy patients, especially in children [3-5]. It is known to occur frequently at the tracheostomy stoma, where the tracheostomy site meets the skin [6]. Treatment options include non-surgical methods such as topical application of antibiotic or steroid ointments, steroid inhalation therapy, or surgical excision using laser or electrocautery [3].
Triamcinolone acetonide is an intermediate-acting glucocorticoid agent that has anti-inflammatory and immunosuppressive effects [7]. Local triamcinolone acetonide injection (TRIAM) is used in various otorhinolaryngologic areas such as preauricular sinus, nasal polyps, allergic rhinitis, benign vocal cord lesions, oral lichen planus, and tracheal stenosis [8-11], and reports of its use in the treatment of granulation tissue in the trachea have also been published [12]. It has also been used in other specialties for various skin lesions such as slow-healing surgical or traumatic wounds, skin graft donor sites, and keloid scars [13,14].
We thus conducted a study to investigate whether the same treatment method could be used for granulomatous tissue on the tracheocutaneous stoma and report the findings along with a literature review, as we experienced improvement of granulation tissue without surgical excision.
Subjects and MethodsPatientsFrom January 2018 to June 2019, we treated patients who were referred to the otorhinolaryngology department for treatment of proliferative granulation tissue around the tracheostomy site among patients admitted to the hospital and had a long-term tracheostomy tube. Among the patients, 20 patients who had proliferative granulation tissue on both sides around the tracheostomy site were selected and their medical records were retrospectively analyzed. Patients themselves or patients with guardians who did not grant consent to the treatment, those who were in poor physical condition due to decreased consciousness or infectious diseases requiring isolation, and those who could not have the tube removed temporarily due to mechanical ventilation were excluded from the study. The Institutional Review Board of the University Hospital approved this study (IRB No. 2019-07-012).
Treatment protocolPrior to administrating TRIAM, all patients received povidone iodine dressing and mupirocin ointment (Esroban, JW Pharm Co., Seoul, South Korea) treatment three times a day for one week as conventional treatment. Then, patients who received conventional treatment but showed no significant change in the surface area of granulomatous tissue were additionally treated with TRIAM. Triamcinolone acetonide (Shin Poong Pharm Co., Seoul, South Korea) was used, and considering its half-life of 24-36 hours [15]. There were variations in treatment dosage, frequency, and duration of intralesional triamcinolone injection according to research [16,17], but due to the lack of prior research on the treatment of granulation tissue on tracheocutaneous stoma, considering the half-life, a total of three injections were administered with an interval of approximately 36-48 hours over one week. The injection was not administered too deep to penetrate the granulomatous tissue itself, and a 1 mL syringe with a 26-gauge needle was used to inject into the soft tissue around the granulomatous tissue [13]. After removing the tracheostomy tube, the injection was administered, and a suction device was used during injection to prevent the triamcinolone acetonide solution from being aspirated. The injection dose was approximately 0.2-0.3 mL per injection [13].
Outcome assessmentTo evaluate the change in tissue, a total of 3 photographs of the lesion were taken: before any treatment, before the first injection (after conventional treatment), and 7 days after the first injection, with a ruler in centimeters placed in the photographs for reference (Fig. 1). The surface area of the tissue was measured using Image J (Rasband, W.S., Image J; U.S. National Institutes of Health, Bethesda, Maryland, USA; https://imagej.nih.gov/ij/, 1997-2018.) by setting 1 cm of the ruler in the photograph as the standard and measuring the area.
Statistical analysisStatistical analysis was performed using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) to compare the difference in the surface area of granulomatous tissue in patients after conventional treatment and the surface area after TRIAM treatment. Paired t-tests were used for analysis after Kolmogorov-Smirnov normality testing. All statistical significance was considered to be p<0.05.
ResultsA total of 20 patients consisting of 12 males and 8 females were included, with an average age of 60.0±14.3 years. The initial surface area of granulation tissue was 1.266±0.449 cm2, and 1.243±0.432 cm2 after conventional treatment, showing no statistically significant difference in tissue area (p=0.143) (Table 1). The pre-injection surface area of the granulation tissue was 1.243±0.432 cm2, and the area on the 7th day after the third injection was 0.477±0.217 cm2, showing a significant difference (p<0.001), thus confirming the therapeutic effect of intralesional triamcinolone injection (Fig. 2 and Table 2).
DiscussionThrough this study, it was confirmed that TRIAM is rapid and effective in treating granulomatous tissue on tracheocutaneous stoma. While there was no significant reduction in the surface area of granulomatous tissue between pre- and post-conventional treatments (p=0.143) (Table 1), a significant decrease in granulomatous tissue was observed after a total of 3 injections when compared to pre-injection levels (p<0.001) (Table 2), confirming the effectiveness of local triamcinolone injection. According to the authors’ investigation, this study presents a novel and straightforward non-surgical method using intralesional triamcinolone injection in treating granulomatous tissue on tracheocutaneous stoma.
Patients who undergo tracheostomy due to acute upper airway obstruction typically undergo decannulation and tracheostomy closure surgery within 2 weeks after the surgery. However, if natural breathing through the upper airway is difficult due to other causes or the patient requires mechanical ventilation, the tracheostomy site may be maintained for several months to years. The incidence of complications of tracheostomy varies depending on the time of onset after surgery and has been reported to be between 5% and 40% [1]. Acute complications include bleeding, infection, pneumothorax, accidental extubation, etc. [2], and delayed complications include granulation tissue, tracheal stenosis, and bleeding [3]. Granulation tissue, which is the most common but non-lethal complication among delayed complications, is a polyp-shaped tissue with developed blood vessels that occurs during the proliferation stage of inflammation around the skin of the tracheostomy site. If the tissue grows into the inside of the trachea, it can lead to tracheal stenosis or obstruction [6]. The cause is thought to be due to bacterial infection with skin flora (such as Staphylococcus epidermidis) that accumulates around the fistula and physical friction between the skin and tracheostomy tube, sutures, as well as powders from surgical gloves. It most commonly occurs in the upper part of the internal orifice where the tracheal wall meets the tracheostomy site and also easily occurs at the external orifice where the skin meets the tracheostomy site [5,6,18].
Among the existing treatment methods, non-surgical methods such as steroid ointments, antibiotic ointments, nitric acid solutions, and steroid inhalation therapy have been used. Surgical methods such as electrocautery, surgical resection, and laser have also been performed, and bronchoscopic resection for intra-tracheal granulation tissues [3,6]. However, the existing non-surgical methods have not shown clear efficacy, with frequent recurrence, while surgical methods have the disadvantage of possible complications such as bleeding and aspiration, and in some cases, the need for general anesthesia [6]. Likewise, conventional treatment for one week did not show statistically significant improvements in this study (Table 1), and as TRIAM does not require general anesthesia but only a 1 mL syringe, there was no bleeding after injection. Also, aspiration could be prevented as suction was also available during the procedure. In addition, there was a significant decrease in granulation tissue after the injection therapy (Table 2), and no recurrence was observed in all patients for 2 weeks. In this study, to prevent aspiration due to injection fluid leakage, the cannula was removed and suction was performed during injection. However, as no aspiration occurred during the procedure, it is anticipated that injections can be administered in the future while the cannula is in place. This is expected to reduce discomfort for the patients.
Local triamcinolone injection is mainly used to treat keloids and hypertrophic scars, as it suppresses the expression of keloid fibroblast and vascular endothelial growth factor in the tissue and induces apoptosis of fibroblasts, causing atrophy of fibrous tissue [19]. Local triamcinolone injection is known to be effective not only for granulation tissue treatment but also for reducing treatment period and cost [13], and can also be expected to relieve pain caused by inflammation [20].
Although local triamcinolone injection has the advantage of lower chance of systemic toxicity than systemic steroid injection or oral medication, there are also disadvantages. Local atrophy, discoloration, staining, and telangiectasia may occur at the injection site, and direct injection into major blood vessels may cause systemic side effects. Furthermore, although rare, permanent vision loss has also been reported [14,21]. To avoid such complications, injection was targeted in areas around the tracheostomy avoiding major blood vessels, and side effects were not observed in this study.
Finally, the limitations of this study were that the number of patients included was small, with only 20 patients, and that only the surface area of the granulomatous tissue was analyzed. In addition, there was a lack of analysis on detailed medical histories, such as degree of pain, duration of tracheostomy status, and previous use of antibiotics, patient satisfaction in comparison to conventional methods, etc. Another limitation is the inability to compare the effectiveness based on the duration and frequency of TRIAM. While periodic observations were made for up to 2 weeks after the third injection, most patients were unable to undergo long-term follow-up observation due to hospital discharge, transfer, or tracheostomy closure surgery. Consequently, the number of patients with complete resolution could not be measured. Also, the fact that only the surface area was measured in a two-dimensional manner and not in a three-dimensional manner is expected to have affected the accuracy of the effect assessment. Therefore, in the future, it will be necessary to conduct larger-scale studies with a control group and an injection group, and to perform long-term follow-up observations to evaluate treatment effects and prognosis more accurately. Furthermore, through research comparing the effects based on the duration and frequency of TRIAM, it is expected that an appropriate guideline can be established, allowing for the determination of treatment intervals. This is anticipated to reduce inconvenience for outpatient visits due to frequent visits.
In conclusion, this study suggests that TRIAM can be considered as a simple, fast and effective non-surgical treatment option for granulomatous tissue on tracheocutaneous stoma.
NotesAuthor contributions Conceptualization: Dong Sik Chang. Data curation: Min Woo Kim, Myung Ho Jin, Jong Kwan Kim. Formal analysis: Min Woo Kim. Methodology: Min Woo Kim. Visualization: Min Woo Kim. Writing—original draft: Min Woo Kim. Writing—review & editing: Min Woo Kim, Dong Sik Chang. Fig. 2.Photos of granulomatous tissue on tracheocutaneous stoma before and after injection. A: Pre-injection. B: Post-injection. 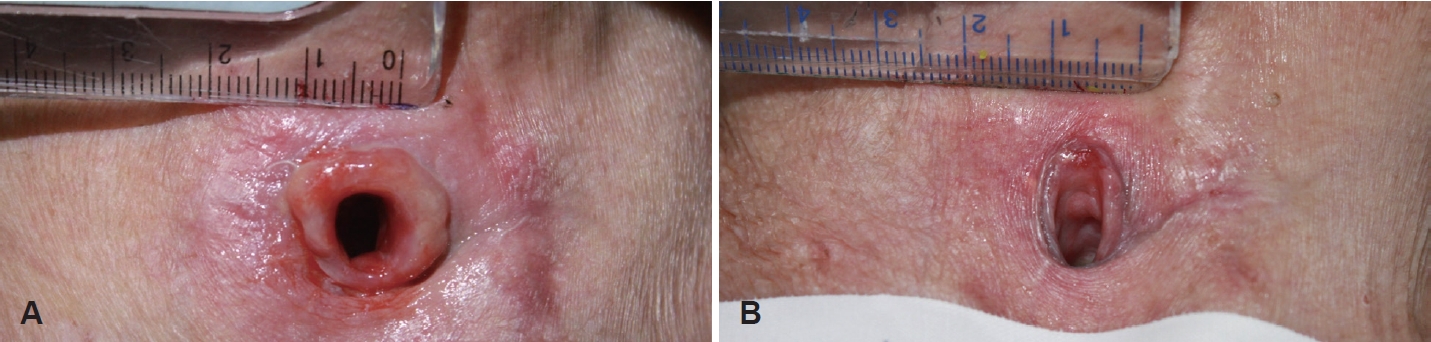
Table 1.The difference in surface area of granulomatous tissue on tracheostomy stoma before and after conventional treatment (n=20)
REFERENCES1. Cheung NH, Napolitano LM. Tracheostomy: epidemiology, indications, timing, technique, and outcomes. Respir Care 2014;59(6):895-915, discussion 916-9.
4. Goldenberg D, Ari EG, Golz A, Danino J, Netzer A, Joachims HZ. Tracheotomy complications: a retrospective study of 1130 cases. Otolaryngol Head Neck Surg 2000;123(4):495-500.
5. Rosenfeld RM, Stool SE. Should granulomas be excised in children with long-term tracheotomy? Arch Otolaryngol Head Neck Surg 1992;118(12):1323-7.
6. Yaremchuk K. Regular tracheostomy tube changes to prevent formation of granulation tissue. Laryngoscope 2003;113(1):1-10.
7. Abraham G, Demiraj F, Ungemach FR. Comparison of the hypothalamic-pituitary-adrenal axis susceptibility upon single-dose i.m. depot versus long-acting i.v. triamcinolone acetonide therapy: a direct pharmacokinetic correlation. J Endocrinol 2006;191(2):491-6.
8. Chang DS, Lee HY, Choi MS, Song K, Kim AY, Cho CS. Intralesional triamcinolone injections for the treatment of preauricular sinus infections. Am J Otolaryngol 2016;37(6):523-7.
9. Wang CT, Lai MS, Hsiao TY. Comprehensive outcome researches of intralesional steroid injection on benign vocal fold lesions. J Voice 2015;29(5):578-87.
10. Lee YC, Shin SY, Kim SW, Eun YG. Intralesional injection versus mouth rinse of triamcinolone acetonide in oral lichen planus: a randomized controlled study. Otolaryngol Head Neck Surg 2013;148(3):443-9.
11. Kapucu B, Cekin E, Erkul BE, Cincik H, Gungor A, Berber U. The effects of systemic, topical, and intralesional steroid treatments on apoptosis level of nasal polyps. Otolaryngol Head Neck Surg 2012;147(3):563-7.
12. Takayama T, Kotani I, Kurosawa T, Yoshimura H. Post-tracheotomy intratracheal granulation tissue response to local injections of triamcinolone. J Bronchology 2001;8(1):29-31.
13. Moio M, Mataro I, Accardo G, Canta L, Schonauer F. Treatment of hypergranulation tissue with intralesional injection of corticosteroids: preliminary results. J Plast Reconstr Aesthet Surg 2014;67(6):e167-8.
14. Muneuchi G, Suzuki S, Onodera M, Ito O, Hata Y, Igawa HH. Long-term outcome of intralesional injection of triamcinolone acetonide for the treatment of keloid scars in Asian patients. Scand J Plast Reconstr Surg Hand Surg 2006;40(2):111-6.
16. Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol 2018;11:387-96.
17. Zhang Y, Li S. Transcutaneous injection of triamcinolone acetonide for persistent glottic granulation after laser microsurgery. Braz J Otorhinolaryngol 2023;89(3):359-65.
18. Gupta A, Cotton RT, Rutter MJ. Pediatric suprastomal granuloma: management and treatment. Otolaryngol Head Neck Surg 2004;131(1):21-5.
19. Wu WS, Wang FS, Yang KD, Huang CC, Kuo YR. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol 2006;126(6):1264-71.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |