비대칭성 감각신경성 난청에서 안진 기반의 전정신경초종 선별 검사
Nystagmus Based Screening System for Vestibular Schwannoma in Patients With Asymmetric Hearing Loss
Article information
Trans Abstract
Background and Objectives
We conducted this study to propose a nystagmus based screening system (NBSS) for detecting vestibular schwannoma (VS) in patients with asymmetric sensorineural hearing loss (ASNHL).
Subjects and Method
Data of 90 patients diagnosed with VS were retrospectively analysed. For the 1:1 allocation, 90 ASNHL patients who were not diagnosed with VS were sequentially enrolled as the non-VS group and retrospectively analysed. Every participant received pure tone audiometry (PTA), a bedside vestibular function test, and magnetic resonance imaging. A total of 122 patients who passed the exclusion criteria were selected for the study.
Results
The sensitivity and specificity of the abnormal criteria of auditory brainstem response (ABR) for the detection of VS in patients (n=70) showing analyzable wave response were 80.0% and 52.0%, respectively. However, when we included all of the patients (n=122) who did not show analyzable wave response in ABR, the sensitivity and specificity of the abnormal criteria decreased to 34.8% and 68.4%, respectively. NBSS is composed of vertical spontaneous nystagmus, direction changing gaze evoked nystagmus, ipsilesional monophasic type head shaking nystagmus (HSN), and ipsilesional to contralesional biphasic type HSN. The sensitivity and specificity of the NBSS were 56.5% and 82.9%, respectively.
Conclusion
NBSS may be used as a supplementary test to ABR in detecting VS of ASNHL.
Introduction
Vestibular schwannoma (VS) is the most common (>90%) benign cerebellopontine angle tumor originating from Schwann cells of the 8th cranial nerve (vestibulocochlear nerve). The incidence of VS is 19/1000000 per year, showing no sexual prevalence [1,2]. Over the last four decades, the incidence of VS has increased and the size of the tumor at the time of diagnosis tends to decrease thanks to familiar usage of radiographic imaging for clinical purpose [2,3]. Although MRI shows great sensitivity for VS, it is difficult to use it as a screening test because of its high financial burden and time consuming procedure. Moreover, the report that only 3% of asymmetric sensorineural hearing loss (ASNHL) patients who receive MRI are diagnosed to VS raises a question about the practicality of MRI [4].
Since the late 1970s, the efficacy of auditory brainstem response (ABR) has been supported by many studies. Koors, et al. [5] have performed a meta-analysis of ABR in VS and calculated that the average sensitivity of ABR is 93.4% with an average specificity of 82%, supporting that ABR is a useful diagnostic tool for patients with clinically suspected VS. However, a significant number of patients with high level of hearing loss did not show wave response after auditory stimulation [6-10]. Moreover, for small intracanalicular tumors, ABR showed noteworthy false negative outcomes [6]. Therefore, a new screening test for VS in ASNHL patients which can also be applied to patients with severe to profound hearing loss without false negative, which is easy to perform, and which is cost-effective, is needed.
It is generally accepted that head shaking nystagmus (HSN) is caused by an asymmetrical peripheral vestibular input or an overcompensation of central velocity storage mechanism [11]. HSN can be biphasic sometimes, meaning that the direction of nystagmus after head shaking changes before the nystagmus disappears. After the first announcement of the presence of biphasic HSN [12], generally accepted assumption of biphasic HSN is that it is a result of prolonged vestibular adaption after completion of peripheral vestibular adaption or by central vestibular disturbance [11,13]. Although there are some reports of HSN beating toward lesional side primarily after head shaking, no single satisfactory explanation of this phenomenon has been presented [14-20].
Herein, the authors devised a nystagmus based screening system (NBSS) for VS in ASNHL patients. The NBSS is composed of four different types of nystagmus: vertical spontaneous nystagmus (SN) and direction changing gaze evoked nystagmus (GEN), which are previously studied central sign nystagmus [21-24], and two types of HSN which firstly show ipsilesional nystagmus after head shaking. We also compared the sensitivity and specificity of NBSS with ABR and confirmed the usefulness of the new screening system.
Subjects and Methods
Subjects
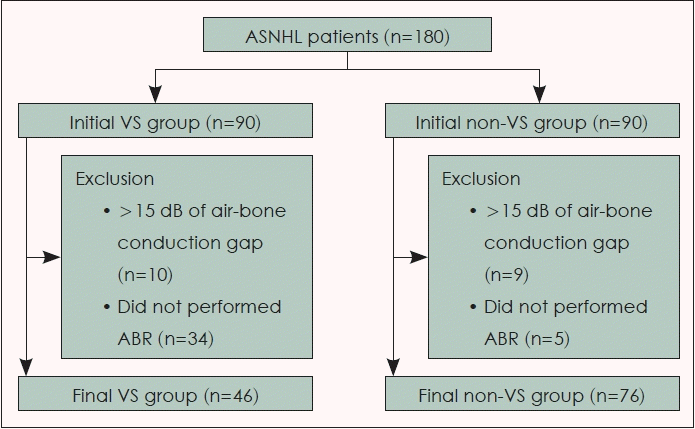
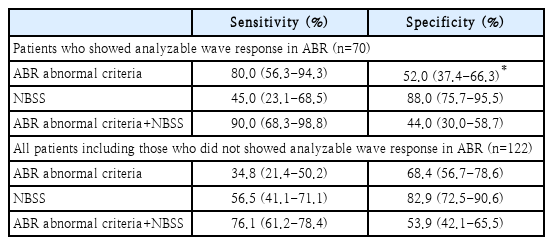
Data of patients in our tertiary referral hospital with asymmetric hearing loss who were confirmed to have ASNHL by pure tone audiometry (PTA) and diagnosed with VS after MRI and/or pathological confirmation after surgery from July 1, 2006 to December 31, 2021 were retrospectively analyzed. We excluded patients who showed more than 15 dB of air-bone conduction gap in PTA and who did not undergo ABR evaluation. For the 1:1 allocation, 90 ASNHL patients who did not showed any evidence of central lesion in MRI and diagnosed to peripheral ASNHL from May 1, 2020 to January 15, 2022 were sequentially and retrospectively enrolled for the non-VS group. Among 90 patients of the VS group, 46 patients were left after exclusion. For the allocated non-VS group, 76 patients were left after applying the same exclusion criteria (Fig. 1).
Data collection
All patients underwent detailed otologic examinations including clinical history, otoscope exam, PTA, and bedside vestibular function test. All PTA data were collected at the time of first visit, and vestibular function test data were collected within two weeks of the first visit. Seven different frequencies (250, 500, 1000, 2000, 3000, 4000, and 8000 Hz) for air conduction and six different frequencies (250, 500, 1000, 2000, 3000, 4000 Hz) for bone conduction test were collected. The threshold of hearing was calculated by the standard hexadecimal method [25]. Patient’s word recognition score (WRS) was confirmed by Korean standard monosyllabic word list for adults [26]. Nystagmus data were recorded and interpreted using videonystagmography. Every patient who was enrolled in this study received internal auditory canal MRI within six months after the first visit for asymmetric hearing loss. This study was approved by the Institutional Review Board of the Kangbuk Samsung Hospital (IRB File No. 2022-08-056), and the requirement of informed consent was waived.
HSN
A positive HSN result was defined as the presence of at least 3 beats of nystagmus after 20 cycles of passive head rotation at a 2 Hz rate with 30 degrees of head tilting forward in the plane of horizontal semicircular canal. We recorded the data for at least one minute after ceasing of the primary nystagmus, not to miss the biphasic nystagmus after a long latency. The SN and GEN was recorded simultaneously.
We categorized the HSN into four different types: I-Mono (ipsilesional monophasic), C-Mono (contralesional monophasic), IC-Bi (ipsilesional to contralesional biphasic), and CI-Bi (contralesional to ipsilesional biphasic). I-Mono meant that the beating occurred to ipsilesional side after head shaking test without direction changing. The opposite was true in C-Mono. IC-Bi meant that the beating primarily occurred to the ipsilesional side followed by change of direction toward the contralesional side. The opposite was true in CI-Bi [7,27].
ABR
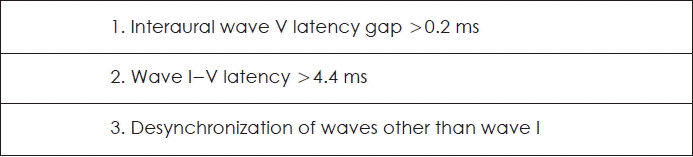
ABR (Bio-logic Navigator PRO; Natus, San Carlos, CA, USA) stimuli were applied by click sound of 90 dB SPL under the 13 rate of sounds per second with 1024 sum of sound setting. Active and ground electrodes were placed on forehead. Reference electrodes were placed on both mastoid processes for each. Abnormal criteria are shown in the Fig. 2 [6]. When the stable waveform of V did not appear under the 90 dB SPL stimulation, the result was considered to be non-measurable.
Statistical analysis
The chi-square test was used for calculating sensitivity and specificity of ABR and bedside vestibular exam. McNemar’s test was used to compare diagnostic performances of ABR and the NBSS. All statistical analyses were performed using Windows SPSS version 24.0 (IBM Corp., Armonk, NY, USA) and a 5% significance level was considered.
Results
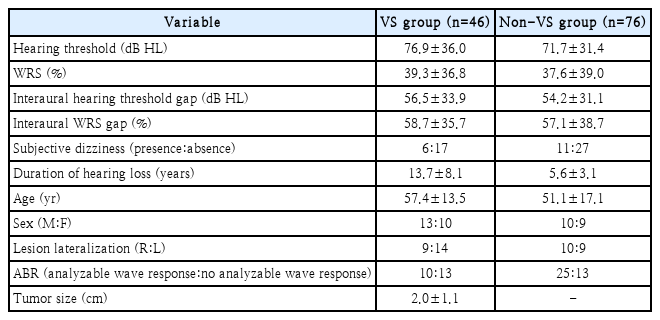
Of 90 patients in the VS group and 90 patients in the non-VS group, 46 and 76 patients passed our exclusion criteria, respectively. PTA, WRS, ABR, bedside vestibular exam (SN, GEN, and HST), and internal auditory canal MRI were performed for every enrolled patient. All data were retrospectively analysed. Table 1 shows demographic and clinical data of patients.
PTA and WRS
Hearing threshold and WRS in the VS group were (mean±standard deviation [SD]) 76.9±36.0 dB HL and 39.3%±36.8%, respectively. In the non-VS group, hearing threshold and WRS were 71.7±31.4 dB HL and 37.6%±39.0%, respectively. There was no statistically significant difference in hearing threshold or WRS between the two groups (p>0.05). Interaural hearing threshold and WRS gap were 56.5±33.9 dB HL and 58.7%±35.7% for the VS group and 54.2±31.1 dB HL and 57.1%±38.7 % for the non-VS group, respectively. There was also no significant difference in interaural hearing threshold or WRS gap between the two groups either.
Subjective dizziness and duration of hearing loss
Twelve (26.1%) patients in VS group and 22 (28.9%) patients in non-VS group had subjective dizziness at the time of evaluation. There was no significant difference in the presence of subjective dizziness between the two groups. The mean value and SD of duration of hearing loss in VS and non-VS group were 13.7±8.1 years and 5.6±3.1 years, respectively. There was significant difference in the duration of hearing loss between the two groups (p<0.001).
ABR
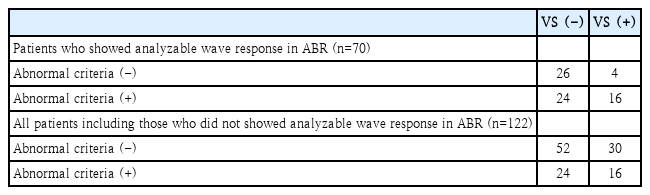
Among the enrolled 122 patients, 70 (57.4%) patients showed analyzable wave response in ABR. However, remained 52 (42.6%) patients did not show any analyzable wave response in ABR. The mean value and SD of hearing threshold was 53.2±24.9 dB for those who showed analyzable wave response in ABR. For those who did not showed analyzable wave response in ABR, the mean value and SD of hearing threshold was 101.1±33.1 dB. Among the 46 VS patients, 20 (43.5%) showed analyzable wave response in ABR while 26 (56.5%) did not. Among the 76 non-VS patients, 50 (65.8%) showed analyzable wave response in ABR while 26 (34.2%) did not. There was a statistically significant (p=0.023) difference in the presence of analyzable wave response between the two groups. In other words, significant number of VS patients did not give us a chance to apply the abnormal criteria of ABR because of the absence of wave response. When we selectively applied the abnormal ABR criteria to patients with those who showed analyzable wave response in ABR, calculated sensitivity and specificity for VS were 80.0% and 52.0%, respectively. However, when we enrolled all patients including those who did not showed an analyzable wave response in ABR, the abnormal ABR criteria’s sensitivity decreased to 34.8%, while its specificity increased to 68.4% (Fig. 3 and Table 2).
ABR result in VS and non-VS group. A: Total patient. B: VS group. C: Non-VS group. PTA, pure tone audiometry; VS, vestibular schwannoma.
NBSS
Eleven patients showed vertical SN or direction changing GEN in our study. All of them were in the VS group. Of the 46 patients in the VS group, 39 (84.8%) patients showed presence of nystagmus after head shaking test, including all of those who showed vertical SN or direction changing GEN (11 patients). Of the 76 patients in the non-VS group, 37 (48.7%) patients showed presence of nystagmus after head shaking test. The calculated sensitivity and specificity for the prevalence of HSN response were 84.8% and 51.3%, respectively. When we evaluated patients who showed any of vertical SN, direction changing GEN, I-Mono, or IC-Bi after the head shaking test (NBSS, as we previously nominated), 26 of 46 patients with VS were screened. The calculated sensitivity and specificity were 56.5% and 82.9%, respectively. The NBSS showed a diagnostic performance equal to that of ABR when we compared in the every enrolled patients (n=122). Moreover, it showed better specificity (88.0%) compared to abnormal criteria of ABR (52.0%) in those who showed analyzable wave response in ABR (n=70). When we applied the abnormal criteria of ABR and NBSS simultaneously, the calculated sensitivity and specificity were 90.0% and 44.0% for patients who showed analyzable wave response in ABR (n=70), respectively. The sensitivity and specificity of simultaneous application were 76.1% and 53.9%, respectively, for every enrolled patient including those without analysable wave response ABR (n=122) (Table 3).
Severe to profound ASNHL (hearing threshold >70 dB)
A total of 64 patients in our study had severe to profound hearing loss (>70 dB of hearing loss). Even under the maximal 90 dB SPL of stimuli, they showed low response rate to the ABR. Only 16 (25%) patients showed a valid I and V wave. Of them, 13 were screened by the abnormal criteria of ABR. However, none of them was confirmed to have VS.
In our NBSS, there were no dropped out cases because this test was independent of individual’s hearing threshold. Of the 64 patients, 24 patients were screened by the NBSS and 15 of 24 patients were confirmed to VS. The calculated sensitivity and specificity of the NBSS in patients with severe to profound hearing loss were 62.5% and 80%, respectively. According to our results, our NBSS is more useful than ABR, especially in patients with severe to profound hearing loss. Authors present a case of a VS patient with profound hearing loss who did not show wave response in ABR but detected by the NBSS (Fig. 4).
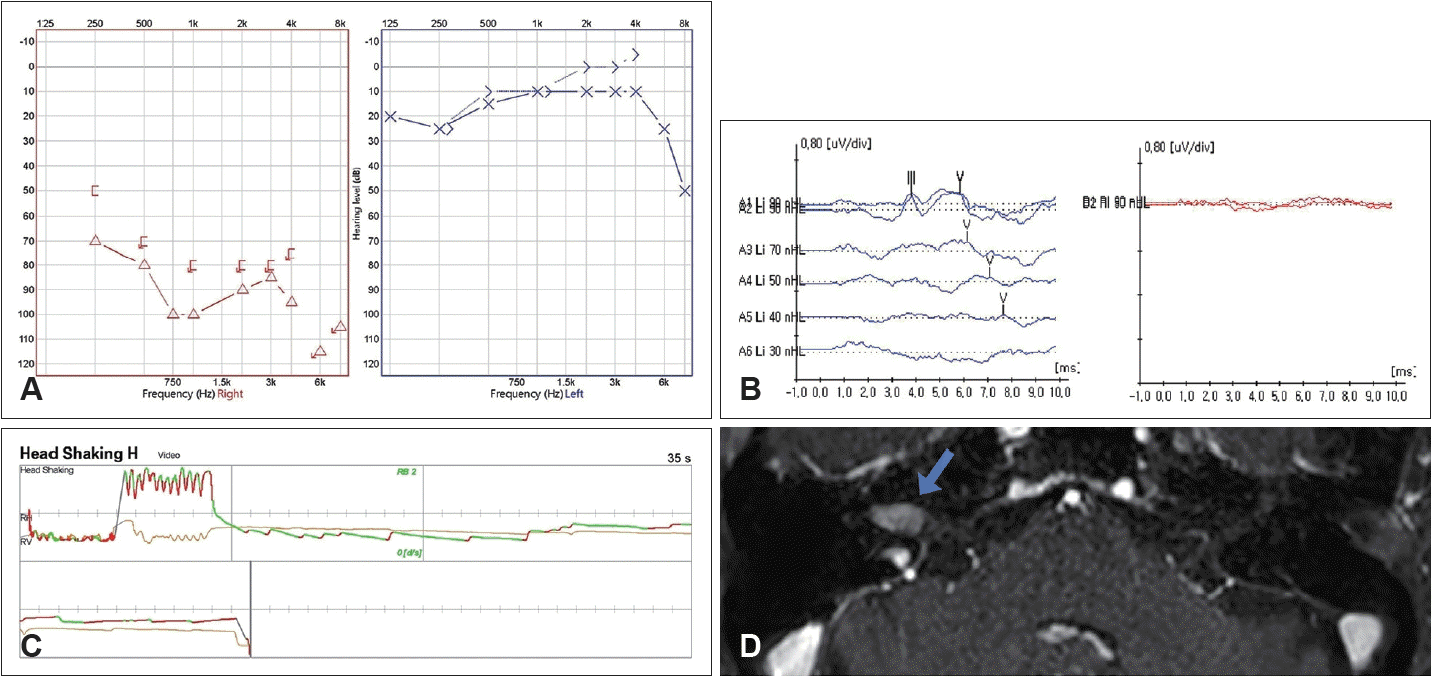
A case of VS detected by the NBSS. A case of VS detected by the NBSS. A case of 66-year-old female who visited our clinic with progressive Rt hearing loss. A: Hearing threshold of air conduction in Rt ear was 93 dB. B: There was no analyzable wave response in ABR exam at the Rt ear. C: The patient showed ipsilesional monophasic type head shaking nystagmus in our bedside screening system as an expression of central sign to detect VS. D: She was finally diagnosed with intracanal VS after MRI. Blue arrow: gadolinium enhancing mass in Rt internal auditory canal, suggesting vestibular schwannoma. VS, vestibular schwannoma; NBSS, nystagmus based screening system; Rt, right; ABR, auditory brainstem response.
Discussion
Because the VS usually grows slowly and gradually compresses the nearby structure, there is a sufficient time for human’s vestibular compensation system to fully adjust. This might explain the reason why there is a significant number of patients with VS complain of asymmetric hearing loss rather than acute exacerbation of dizziness. However, since the vestibule-cochlear nerve is an origin of the tumor, there should always be an inherent vestibular dysfunction in patients with VS. The dysfunction might be the result of long-term accumulation of vestibular compensation. Inspired by the concept of interpreting biphasic HSN with adaptation in vestibular nerve [11], it has been proposed that larger loss of vestibular function promotes greater neural adaptation which would be shown as strong compensatory nystagmus after a head shaking test [27]. By extending the previous idea, the authors hypothesized that in a situation when vestibular compensation had been steadily progressed for a long time, the momentary potential of compensation would also increase and the result would be seen as a strong immediate release of excitatory stimuli by ipsilesional side after head shaking. We thought that this concept of vestibular overcompensation could also be applied to VS. Furthermore, to not miss a certain central sign, we included vertical nystagmus and direction changing GEN in our screening system which are known to be the nystagmus occurring by disorganization of neural integrator or impairment of cerebellar gaze-holding mechanism [21,22].
In our study, sensitivity and specificity of presence of nystagmus after head shaking test (positive HSN) were 84.8% and 51.3%, respectively. They were significantly different from previous studies of HSN [14,15], which proposed lower sensitivity of HSN for detecting VS. Humphriss, et al. [14] have reported a sensitivity of 22% as a result of 22 positive HSN among 102 subjects. Asawavichiangianda, et al. [15] have reported a sensitivity of 47.6% calculated by 10 positive HSN among 21 subjects. This discrepancy might be explained by the fact that our study considered positive HSN as the occurrence of more than three beats of nystagmus without any lower limit of degree, while previous studies defined a positive HSN as 5 or more beats with at least 3 degree of slow phase velocity [14] or when at least 5 degree of slow phase velocity occurred [15]. Moreover, no study has suggested the high prevalence of ipsilesional nystagmus (I-Mono and IC-Bi) in VS like us (17 of 39 patients, 43.6%). Rather, the ipsilesional nystagmus was considered as a minor phenomenon violating the Ewald’s second law [14-20]. This difference might have occurred by the setting of the patient that we included in our study. Unlike previous studies which enrolled patients suffering from dizziness, we selected patients who complained of ASNHL regardless of the prevalence of dizziness. Because the majority of the VS arises from vestibular portion of 8th cranial nerve [28,29], those with subjectively detected unilateral hearing loss could be interpreted that the tumor had advanced enough to press the cochlear nerve. Because the VS is a slow growing tumor, it would take a long period for the cochlear nerve to be compressed. Thus, it would have provided sufficient time for vestibular compensation to be accumulated. This inference could explain the high rate of ipsilesional nystagmus by vestibular overcompensation after head shaking in ASNHL patients. Moreover, considering that only 31.8% (7 out of 22) of patients who showed ipsilesional nystagmus after head shaking had subjective dizziness at the time when asymmetric hearing loss was recognized, we assume that vestibular overcompensation could mask the subjective dizziness in VS patients. It could also explain the discrepancy of our result of patients with ASNHL from previous dizziness targeting studies [15-20].
In the patients who showed analysable wave response in ABR, the NBSS alone showed lower sensitivity (45%) than ABR (80%). The ABR has better performance in screening VS compare to NBSS alone when there is a presence of wave response at which the abnormal ABR criteria is applicable. The NBSS alone showed low sensitivity in screening VS. Though we hypothesized the increased momentary potential of vestibular compensation in VS patients, still there were many patients of VS who showed contralateral HSN after head shaking. We expected that momentary potential of vestibular compensation would be increase proportionately to the long term accumulation of vestibular compensation, but this result implies that there might be some other mechanisms affecting the vestibular compensation potential.
When we applied ABR and NBSS together in the patients who showed analysable wave response in ABR, the specificity was the lowest (44%) compared to ABR (52%) and NBSS (88%) alone. Previous study of ABR’s diagnostic performance in VS also showed low specificity (10%-50%) of the ABR [5]. Lowest (44%) result of specificity in combination of ABR and NBSS implies that NBSS was not able to screen the non-VS patient who showed abnormal responses in ABR. There is no single clear explanation to support this, but since the vestibule and cochlear system is not independent pathways, it is acceptable that test using cochlear response (ABR) and vestibular response (NBSS) were not complementary to each other. We would like to emphasize that the strength of NBSS are mainly in the situation when the physician cannot utilize the patients’ auditory system, like the patients with severe to profound hearing loss, as we mentioned above. However, such interpretation should be cautious because there is not enough data of patients and absence of normal control group in this study.
We confirmed that our NBSS could be used as a helpful supplementary material for ABR in detecting ASNHL caused by VS. Additionally, our data showed that a significant number of patients visiting the clinic who were finally diagnosed as VS had severe to profound hearing loss. The ABR was insufficient to screen patients with severe to profound hearing loss, because there was a high rate of absent wave responses after sufficient acoustic stimuli. Our NBSS can be a useful alternative screening method for patients with severe to profound hearing loss without dropped-out cases. However, since there are diverse abnormal criteria of ABR for detecting VS [5], evaluating the efficacy of ABR by single abnormal criteria should be interpreted cautiously. Diverse sensitivity and specificity of ABR in detecting VS had been reported under the different abnormal criteria [5]. Moreover, since the ABR showed unsatisfactory results for small and intracanalicular tumors [5,6], number of such cases involved in different studies could be applied as a bias in evaluating the diagnostic performance of ABR.
Concerning the low cost-effectiveness (low diagnostic yield with high financial burden) and time-consuming process of MRI, the need for alternative screening protocol before imaging has been advocated [30]. To the best of our knowledge, no previous study has analyzed biphasic patterns of HSN in VS or proposed a nystagmus based screening tool using the concept of increased momentary excitatory potential in long termed vestibular overcompensation. Considering that the evaluation of vertical SN, direction changing GEN, and HSN could be completed in a single step approach with little financial burden, we believe that the NBSS could practically help many physicians who has hesitation of managing ASNHL patients in their clinics. The highest sensitivity (84.8%) for VS was adopted in positive HSN result. When we applied the abnormal criteria of ABR and NBSS simultaneously, the satisfactory sensitivity and specificity balance (76.1% and 53.9% for each) were obtained. Therefore, the authors recommend to check the HSN first. If the result is positive, then the physician can continue to proceed ABR and/or the NBSS. The physician can utilize our NBSS as a supplementary method to ABR for diagnosing VS in ASNHL patients.
There are some limitation in this study. First, this is a retrospective study using 1:1 allocation of the VS and non-VS group. Considering the low incidence of VS, there is a worrisome of selection bias that severely effecting the result of this study. Second, there is a significant difference in the duration of hearing loss between the VS and non-VS group. Since this study used a concept of the accumulation of vestibular compensation, difference in hearing loss duration might imply different amount of accumulation between the two groups, which could influenced the direction of HSN. Third, sensitivity and specificity of ABR, NBSS and simultaneous application of ABR and NBSS were relatively low in this study. Therefore, the efficacy of NBSS as a screening tool for VS could be debatable. However, the advantage of this study is in the proposal of nystagmus based screening test for the VS. For the patients with severe to profound hearing loss, ABR seemed unsatisfactory for screening because of the high rate (75%) of absent wave responses. However, since the NBSS is independent of individual’s hearing threshold, it could be helpful especially in those without wave responses in ABR.
Acknowledgements
None
Notes
Author Contribution
Conceptualization: Min-Beom Kim. Data curation: Joon-Pyo Hong. Formal analysis: Joon-Pyo Hong, Min-Beom Kim. Investigation: Joon-Pyo Hong, Jung-Yup Lee. Methodology: Joon-Pyo Hong, Jung-Yup Lee. Validation: Jung-Yup Lee, Min-Beom Kim. Writing—original draft: Joon-Pyo Hong. Writing—review & editing: Jung-Yup Lee, Min-Beom Kim.