 |
 |
AbstractBackground and ObjectivesNasal obstruction has been assumed to be correlated with sleep-related breathing disorder (SRBD). However, a definite correlation between nasal obstruction and SRBD is still controversial. This study aimed to define whether symptoms and severity of the deviated septum of nose (DSN) and inferior turbinate hypertrophy (ITH) are correlated with the severity of SRBD.
Subjects and MethodThis is a retrospective study of 60 patientsŌĆÖ preoperative polysomnography who have undergone septoplasty and turbinoplasty. Patients with obesity, tonsillar hypertrophy, high Mallampati class, mandibular problem, and nasal polyp or concha bullosa were excluded from the analysis. Subjective nasal obstruction scores, and DSN/ITH grades were collected, and correlations between apnea-hypopnea index (AHI), lowest oxygen saturation, and snoring time were analyzed.
ResultsThe average of AHI, lowest saturation and relative snoring time were 3.72┬▒5.79, 89.78%┬▒6.81%, and 8.45%┬▒10.43%, respectively. The number of patients who were normal, obstructive sleep apnea (OSA) syndrome mild, moderate, and severe were 49 (82%), 6 (10%), 4 (7%) and 1 (1%), respectively. The degrees of DSN and ITH, and subjective nasal congestion scores showed no significant correlation with polysomnographic results. Age and AHI had a correlation coefficient of 0.54 (p<0.001) and AHI increased with increasing age. Multiple linear regression demonstrated that age (regression coefficient 0.229, 95% confidence interval 0.135 to 0.322, p<0.001) was significantly associated with AHI, while other variables showed no statistically significant association with AHI (p>0.05).
IntroductionSleep-related breathing disorder (SRBD) is abnormal respiration during sleep, ranging from snoring to severe obstructive sleep apnea (OSA). OSA occurs in 4.5% of male and 3.2% of female [1] and is characterized by repeated airway narrowing or collapse during sleep, leading to sleep fragmentation and excessive daytime sleepiness [2,3]. It is known to cause deterioration of sleep-related quality of life and cardiovascular disease, metabolic disorder, cognitive decline, and driving and workplace accidents [4,5]. Snoring is a breathing noise caused by vibration of relaxed upper airway structures, such as the soft palate, uvula, and pharyngeal wall, as the respiratory airflow passes through the narrowed upper airway during sleep, and it occurs in about half of adult males [6]. The cause of SRBD is dysfunction of the upper airway dilator and abnormality of the upper airway structure. It is more common in obesity, old age, and male sex [1,3].
The nasal cavity is the part which airflow first passes through during inhalation and accounts for about 60% of the total upper airway resistance [7]. According to the Starling resistor model, negative pressure is generated in the oropharynx if nasal resistance increases due to nasal obstruction, causing oropharyngeal collapse [8]. Also, oral breathing caused by nasal obstruction is inefficient breathing, increasing upper airway resistance by 2.5 times compared to normal nasal breathing [9]. Furthermore, oral breathing triggers fluttering of the oropharyngeal region, including the soft palate and uvula. It causes the tongue to move backward, reducing the oropharyngeal diameter and aggravating sleep apnea [10]. Such mechanisms could be considered the theoretical basis for nasal obstruction as a significant cause of SRBD.
Clinically, the most common causes of nasal obstruction are deviated septum of nose (DSN) and inferior turbinate hypertrophy (ITH) [11]. Although many patients ask whether DSN and ITH cause SRBD or whether SRBD improves after surgery, we do not have an exact answer yet.
This is because studies have not reached a common conclusion about whether nasal obstruction is an independent causative factor of SRBD. Numerous studies have concluded that nasal obstruction is the cause of SRBD. Still, there were several limitations, such as not controlling the factors that could affect the occurrence of SRBD other than nasal obstruction [12,13], or inducing non-physiologic nasal obstruction through nasal packing [14,15], or relying only on subjective reports on the presence and severity of nasal obstruction [16-18]. In addition, since studies claiming no direct relationship between nasal obstruction and SRBD have been reported [19,20], it has not been concluded whether nasal obstruction alone causes SRBD. In particular, most of the studies exploring the relationship between DSN/ITH and SRBD have determined whether DSN/ITH are present in SRBD patients [21,22]. Therefore, it has not been possible to conclude whether DSN and ITH play roles as independent factors.
Also, since most of the research related to this topic is aimed at Caucasians, there is a possibility that different results may be obtained in Asians due to racial differences. And if DSN/ITH is the independent cause of SRBD, it is necessary to consider further whether the degree of deviation and hypertrophy is correlated with the severity of SRBD.
This study aimed to define whether they are the independent causative factors of SRBD and whether symptoms and severity of DSN/ITH are correlated with the severity of SRBD.
Subjects and MethodsPatientsThis retrospective study included patients who visited Ilsan Paik Hospital, the Republic of Korea, for nasal congestion, were diagnosed with DSN/ITH, underwent septoplasty and turbinoplasty, and performed preoperative polysomnography for screening purpose to detect SRBD from July 2016 to July 2021. This study was approved by the Institutional Review Board of Ilsan Paik Hospital (IRB File No. ISPAIK 2021-10-006). A total of 60 patients was included in this study.
To exclude other anatomical factors that have been reported to be causative for snoring and OSA, patients with body mass index greater than 25, with the degree of tonsil hypertrophy exceeding grade 2 on the Friedman tonsil grading scale, with the position of soft palate and tongue exceeding class 2 on the Modified Mallampati classification, with collapse during muller maneuver, and with mandibular problems such as micrognathia and retrognathia were excluded. Patients with polyp or concha bullosa observed in the nasal cavity were also excluded [21,23,24]. In addition, patients with a history of previous upper respiratory tract surgery, positive pressure device use, sedation, or alcohol abuse and diagnosed with upper respiratory tract infection and sinusitis at the time of polysomnography were excluded.
Nasal congestion measurementsSubjective nasal congestion questionnaires and anterior rhinoscopic findings performed at the time of diagnosis in the outpatient clinic by one experienced rhinologist were reviewed. On their first visit, patients were asked to express the subjective degree of nasal congestion as a score between 0 and 10 using a visual analog scale (VAS). The presence and severity of DSN/ITH were observed through anterior rhinoscopic examination. The degree of DSN was recorded by grading, referring to the method proposed by Jin, et al. [25]. A mild type deviation of less than 50% of the distance from the midline of the nasal septum to the lateral wall of the nasal cavity was measured as 1 point, and if the nasal septum exceeds 50% but does not touch the lateral wall of the nasal cavity is 2 points as the moderate type. The case of contact with the lateral wall was measured as a severe type with 3 points (Fig. 1). The ITH degree was recorded as grade I when only slight hypertrophy was observed without nasal obstruction, grade III when there was complete nasal obstruction due to hypertrophy, and grade II between grades I and III (Fig. 2).
Sleep studiesAll patients have performed polysomnography using Level III Polysomnographic device Embletta (Natus Medical Inc., San Carlos, CA, USA)├Ś10 the day before surgery in the hospital, and the results were reviewed. This is a 9-channel model and consists of a nasal pressure transducer, a temperature sensor, two respiratory motion sensors (chest and abdomen), an oximeter, a posture sensor, and a snoring sensor. Those who slept for more than 300 minutes were included in the study. Apnea and hypopnea were defined as when a decrease of 90% or more of airflow lasted at least 10 seconds, and a decrease of 50% or more of airflow was seen for at least 10 seconds, respectively. The apnea-hypopnea index (AHI) was calculated as the sum of apnea and hypopnea per hour. The lowest saturation during sleep and the relative snoring time, measured by dividing the sleep time during which snoring occurred by the total sleep time, were also recorded.
Data analysisFor statistical analysis, IBM SPSS statistics 23 (IBM Corp., Armonk, NY, USA) was used. The average of AHI, lowest saturation and relative snoring time was compared according to age and sex by independent sample t-test. We analyzed whether DSN/ITH grade and subjective nasal congestion VAS score were correlated with AHI, lowest saturation, and relative snoring time through bivariate correlation analysis. Multiple linear regression analysis was performed to exclude the influence between independent variables when analyzing their association with AHI. Statistical significance was considered when p<0.05.
ResultsOf the total 60 patients, 40 males and 20 females were included. The age ranged from 17 to 75 years, with a mean age of 42.50┬▒18.34 years. As a result of polysomnography, the mean of AHI, lowest saturation, and relative snoring time were 3.72┬▒5.79, 89.78┬▒6.81%, and 8.45┬▒10.43%, respectively. Normal (AHI less than 5), OSA mild (AHI 5 or more and less than 15), moderate (AHI 15 or more and less than 30), severe (AHI 30 or more) were 49 (82%), 6 (10%), 4 (7%) and 1 person (1%), respectively. Relative snoring time was less than 5% in 33 patients (55%) and less than 20% in 50 patients (83%) (Table 1). The mean subjective nasal congestion score was 5.82┬▒1.47 points. The degree of DSN was 16 patients (26.7%) with mild type, 30 patients (50.0%) with moderate type, and 14 patients (23.3%) with severe type, and the degree of ITH was 11 patients (18.3%) with grade I, 36 patients (60.0%) with grade II, and 13 patients (21.7%) with grade III (Table 2).
When comparing the mean of AHI, lowest saturation, and snoring time according to sex, males showed higher AHI, lowest saturation, and snoring time than females, but it was not statistically significant (p=0.671 in AHI, 0.819 in lowest saturation, 0.957 in snoring time, respectively).
Age had a significant correlation with AHI and snoring time, and these values increased with age (SpearmanŌĆÖs p=0.723, p<0.001 in AHI, SpearmanŌĆÖs p=0.365, p=0.004 in snoring time). The lowest saturation also had a correlation with age, showing a tendency to decrease with age (SpearmanŌĆÖs p=-0.623, p<0.001).
Correlation between subjective nasal congestion and polysomnography (PSG) resultsNasal congestion VAS score showed no statistically significant correlation with AHI, the lowest saturation, and snoring time (p=0.132, 0.582, 0.875, respectively).
Correlation between the degree of DSN/ITH and PSG resultsAHI, lowest saturation, and snoring time did not show any statistically significant correlation with DSN and ITH grade, respectively (p>0.05) (Table 3). Each group of DSN mild, moderate, and severe and ITH grades I, II, and III showed no statistically significant difference in the mean value of AHI, lowest saturation, and snoring time.
Multiple linear regression analysis was performed to investigate the independent association between AHI and several factors such as age, DSN, ITH and nasal obstruction score. The age (regression coefficient 0.229, 95% confidence interval 0.135 to 0.322, p<0.001) was significantly associated with AHI, while other variables showed no statistically significant association with AHI (p=0.979 in DSN, p=0.960 in ITH, and p=0.054 in nasal obstruction score).
Comparison between OSA group and non-OSA groupThe subjects were divided into two groups, a non-OSA group (n=49) with an AHI of less than 5 and an OSA group with an AHI of 5 or more (n=11). The mean age of the OSA group was 62.64┬▒10.63, and the non-OSA group was 37.86┬▒ 17.31, and there was a statistically significant difference (p<0.001). The OSA group was male dominant, with sex ratio of OSA group M:F=2.25:1 and non-OSA group 1:1.16 when weighting was applied considering that the sex ratio of the participating patient group was M:F=2:1 (Table 4). There was no significant difference in the DSN/ITH degree of the two groups.
DiscussionNumerous studies on the association between nasal obstruction and SRBD have been conducted. In a sleep study of 1032 people by Young, et al. [26], the incidence of snoring was more than three times higher in the population complaining of nighttime nasal congestion. Lofaso, et al. [27] measured nasal resistance by rhinomanometry and argued that nasal obstruction is an independent risk factor for OSA. Contrary to the above studies, Miljeteig, et al. [19,20] performed PSG and nasal resistance measurements and divided the study population into three groups according to the degree of nasal resistance, and there was no difference in the degree of snoring and OSA between the groups.
For studies on the relationship between DSN/ITH and sleep apnea, Zonato, et al. [28] and de Aguiar Vidigal, et al. [22] found that the frequency of intranasal structural deformities such as septal deviation and turbinate hypertrophy was higher in the sleep apnea patient group than in the normal group.
Studies on whether snoring and sleep apnea improve after nasal septum and turbinate correction have also produced conflicting results. Although there have been studies that snoring improved after septoplasty, pre-and post-operative evaluation was conducted only with a subjective questionnaire [18,29,30]. In a study by Virkkula, et al. [31] that performed rhinomanometry and PSG before and after nasal surgery, nasal resistance decreased after surgery, but sleep apnea and snoring did not improve.
The target group of this study was patients who needed surgical treatment due to DSN/ITH and in the absence of other upper airway anatomical abnormalities that could cause SRBD and other nasal diseases that could cause nasal obstruction. As a result of their sleep test, 82% had no OSA with AHI of 5 or less, and 60% of 18% who had OSA had mild type obstructive sleep apnea syndrome (OSAS) with AHI of 5 or more and less than 15. In the case of relative snoring time, there is no universally used severity threshold like the AHI criteria of OSA, but 33 patients (55%) had a relative snoring time of 5% or less and 50 patients (83%) with 20% or less. In other words, more than 80% of the patients with DSN/ITH included in the study did not have SRBD. Therefore, it can be interpreted that DSN/ITH alone cannot be the causative factor of SRBD.
Age and AHI had a positive correlation, and the AHI increased with age. When patients were classified into the OSA group (n=11) and non-OSA group (n=49), the mean age was 62.64┬▒10.63 and 37.86┬▒17.31, respectively, indicating that the age of the OSA group was significantly higher (p<0.05). Also, the ratio of males was higher in the OSAS group than in the non-OSA group. This was consistent with the higher incidence of SRBD in older adults and males in several previous studies [1]. Therefore, the elderly male patients with DSN and ITH may accompany OSA, but this should be interpreted as a result of the age and sex factor of SRBD, and DSN/ITH should not be considered a causative factor in this case.
Also, there was no statistically significant correlation between the degree of DSN/CHR and PSG results in this study, and neither was the subjective nasal congestion score. Therefore, the state that the degree of DSN/ITH and subjective degree of nasal congestion are severe does not always mean that the severity of sleep apnea or snoring is high.
The authors reasoned as follows as to why DSN and ITH could not be the sole causes of SRBD when other factors that may affect respiratory flow during sleep were excluded. Although nasal obstruction may affect respiration during sleep, it was inferred that various factors such as oropharyngeal instability and backward movement of the tongue should act in combination to cause respiratory airflow disturbance enough to generate snoring and sleep apnea. This is because, in various studies related to the cause of SRBD using dynamic MRI, acoustic analysis, and pressure analysis of the pharyngeal lumen, it is concluded that SRBD does not occur on a single area of the upper airway. Still, it is instead a phenomenon caused by the complex interaction of several areas such as the nasal cavity, oral cavity, nasopharynx, oropharynx, hypopharynx, and larynx [32-36]. In other words, even if nasal resistance increases due to DSN and ITH, if other upper airway structures and functions are normal, compensatory action will occur, and upper airway obstruction will not happen. In addition, since nasal breathing with increased resistance is converted to oral breathing only after a certain threshold is exceeded, and the threshold varies from person to person, the universal correlation between the degree of DSN/ITH and the degree of SRBD could not be established [37].
Recently, since several studies have reported that reversible obstruction caused by allergic rhinitis is more correlated with sleep apnea than fixed nasal obstruction such as DSN and ITH, further research regarding reversible obstruction is needed [38].
The differences between previous studies and this study are as follows. While the previous studies related to DSN/ITH and SRBD mainly investigated whether patients with SRBD accompanied DSN/ITH or whether SRBD improved after correction of DSN/ITH, we investigated whether SRBD was present in patients with DSN/ITH and evaluated only the effects of DSN/ITH by excluding other factors that could cause SRBD. In addition, previous studies mainly correlated the increase in intranasal pressure measured by rhinomanometry or decrease in nasal volume measured by acoustic rhinometry with the degree of nasal obstruction [12,27,39,40]. Since those were the phenomenon induced by the nasal obstruction regardless of its cause, there were limitations in that it did not accurately reflect the presence and severity of DSN/ITH. Therefore, we confirmed the correlation with SRBD through symptom questionnaires and rhinoscopic examinations, standard methods for diagnosing DSN/ITH in clinical practice.
The limitations of this study were that the target group was small and the control group without DSN/ITH was not secured, and an objective test for increased nasal resistance was not performed. In addition, since level IIII polysomnography was conducted instead of level I, the accuracy of test results was limited. Also, since drug-induced sleep endoscopy is now considered a valuable method to evaluate upper airway causes of SRBD, it could not be assured that other upper airway factors were excluded entirely even if we established strict exclusion criteria on physical exams.
In conclusion, more than 80% of patients with DSN/ITH did not have SRBD, and there was no correlation between the degree of DSN/ITH and the degree of SRBD. Therefore, it can be demonstrated that DSN/ITH are not the independent causative factor of SRBD.
NotesAuthor Contribution Conceptualization: Ick Soo Choi. Data curation: Ick Soo Choi. Formal analysis: Ji Min Yun, Yong Seok Jo. Investigation: Ji Min Yun, Yong Seok Jo. Methodology: Yong Seok Jo, Ick Soo Choi. Project administration: Ji Min Yun. Supervision: Ick Soo Choi. Validation: Ick Soo Choi. WritingŌĆöoriginal draft: Ji Min Yun. WritingŌĆöreview & editing: all authors. Fig.┬Ā1.Grading method of septal deviation. A: Mild type of deviation. The vertical dotted line and horizontal dotted line demonstrate the midline of the nasal septum and the distance from the midline to the lateral wall of the nasal cavity, respectively. The arrow indicates the deviation of the nasal septum. Deviation occupies less than 50% of the distance from the midline of the nasal septum to the lateral wall of the nasal cavity. B: Moderate type. Deviation exceeds 50% but does not touch the lateral wall of the nasal cavity. C: Severe type. Deviation contacts with the lateral wall of the nasal cavity. 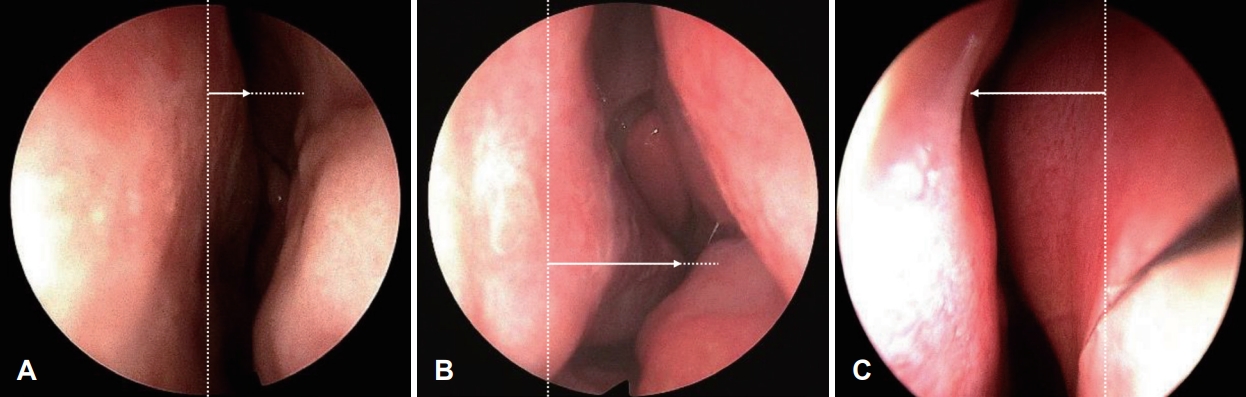
Fig.┬Ā2.Grading method of inferior turbinate hypertrophy. A: Grade I. Inferior turbinate (IT) is slightly hypertrophic without definite nasal obstruction. B: Grade II. IT is hypertrophic but contact with nasal septum is not detected. C: Grade III. IT is hypertrophic and contacts with the nasal septum, causing nearly complete nasal obstruction. 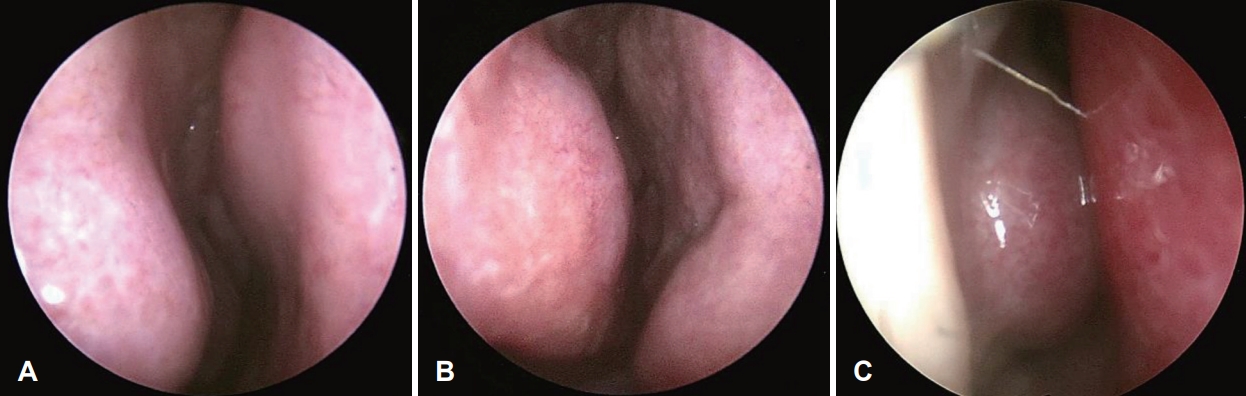
Table┬Ā1.Polysomnographic results of the study population (n=60) Table┬Ā2.Subjective nasal congestion score and DSN/ITH severity of the study population (n=60) REFERENCES1. Kim J, In K, Kim J, You S, Kang K, Shim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med 2004;170(10):1108-13.
3. Azagra-Calero E, Espinar-Escalona E, Barrera-Mora JM, Llamas-Carreras JM, Solano-Reina E. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med Oral Patol Oral Cir Bucal 2012;17(6):e925-9.
4. Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012;5(5):720-8.
5. Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: A meta-review. Respirology 2013;18(1):61-70.
6. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328(17):1230-5.
7. Ferris BG Jr, Mead J, Opie LH. Partitioning of respiratory flow resistance in man. J Appl Physiol 1964;19:653-8.
8. Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol (1985) 1988;64(2):789-95.
9. Fitzpatrick MF, McLean H, Urton AM, Tan A, OŌĆÖDonnell D, Driver HS. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J 2003;22(5):827-32.
10. Meurice JC, Marc I, Carrier G, S├®ri├©s F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med 1996;153(1):255-9.
11. Clark DW, Del Signore AG, Raithatha R, Senior BA. Nasal airway obstruction: Prevalence and anatomic contributors. Ear Nose Throat J 2018;97(6):173-6.
12. Houser SM, Mamikoglu B, Aquino BF, Moinuddin R, Corey JP. Acoustic rhinometry findings in patients with mild sleep apnea. Otolaryngol Head Neck Surg 2002;126(5):475-80.
13. Zwillich CW, Pickett C, Hanson FN, Weil JV. Disturbed sleep and prolonged apnea during nasal obstruction in normal men. Am Rev Respir Dis 1981;124(2):158-60.
14. Olsen KD, Kern EB, Westbrook PR. Sleep and breathing disturbance secondary to nasal obstruction. Otolaryngol Head Neck Surg 1981;89(5):804-10.
15. Suratt PM, Turner BL, Wilhoit SC. Effect of intranasal obstruction on breathing during sleep. Chest 1986;90(3):324-9.
16. Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. J Allergy Clin Immunol 1997;99(2):S757-62.
17. Virkkula P, Bachour A, Hyt├Čnen M, Malmberg H, Salmi T, Maasilta P. Patient- and bed partner-reported symptoms, smoking, and nasal resistance in sleep-disordered breathing. Chest 2005;128(4):2176-82.
18. Ellis PD, Harries ML, Ffowcs Williams JE, Shneerson JM. The relief of snoring by nasal surgery. Clin Otolaryngol Allied Sci 1992;17(6):525-7.
19. Miljeteig H, Hoffstein V, Cole P. The effect of unilateral and bilateral nasal obstruction on snoring and sleep apnea. Laryngoscope 1992;102(10):1150-2.
20. Miljeteig H, Savard P, Mateika S, Cole P, Haight JS, Hoffstein V. Snoring and nasal resistance during sleep. Laryngoscope 1993;103(8):918-23.
21. Zonato AI, Bittencourt LR, Martinho FL, J├║nior JF, Greg├│rio LC, Tufik S. Association of systematic head and neck physical examination with severity of obstructive sleep apnea-hypopnea syndrome. Laryngoscope 2003;113(6):973-80.
22. de Aguiar Vidigal T, Martinho Haddad FL, Greg├│rio LC, Poyares D, Tufik S, Azeredo Bittencourt LR. Subjective, anatomical, and functional nasal evaluation of patients with obstructive sleep apnea syndrome. Sleep Breath 2013;17(1):427-33.
23. Woodson BT, Naganuma H. Comparison of methods of airway evaluation in obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 1999;120(4):460-3.
24. Friedman M, Tanyeri H, La Rosa M, Landsberg R, Vaidyanathan K, Pieri S, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope 1999;109(12):1901-7.
25. Jin HR, Lee JY, Jung WJ. New description method and classification system for septal deviation. J Rhinol 2007;14(1):27-31.
26. Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med 2001;161(12):1514-9.
27. Lofaso F, Coste A, dŌĆÖOrtho MP, Zerah-Lancner F, Delclaux C, Goldenberg F, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J 2000;16(4):639-43.
28. Zonato AI, Martinho FL, Bittencourt LR, de Oliveira Camponês Brasil O, Gregório LC, Tufik S. Head and neck physical examination: Comparison between nonapneic and obstructive sleep apnea patients. Laryngoscope 2005;115(6):1030-4.
29. Low WK. Can snoring relief after nasal septal surgery be predicted? Clin Otolaryngol Allied Sci 1994;19(2):142-4.
30. Li HY, Lee LA, Wang PC, Chen NH, Lin Y, Fang TJ. Nasal surgery for snoring in patients with obstructive sleep apnea. Laryngoscope 2008;118(2):354-9.
31. Virkkula P, Bachour A, Hyt├Čnen M, Salmi T, Malmberg H, Hurmerinta K, et al. Snoring is not relieved by nasal surgery despite improvement in nasal resistance. Chest 2006;129(1):81-7.
32. Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis 1993;148(5):1385-400.
33. Osborne JE, Osman EZ, Hill PD, Lee BV, Sparkes C. A new acoustic method of differentiating palatal from non-palatal snoring. Clin Otolaryngol Allied Sci 1999;24(2):130-3.
34. Kotecha BT, Hannan SA, Khalil HM, Georgalas C, Bailey P. Sleep nasendoscopy: A 10-year retrospective audit study. Eur Arch Otorhinolaryngol 2007;264(11):1361-7.
35. Skatvedt O. Continuous pressure measurements during sleep to localize obstructions in the upper airways in heavy snorers and patients with obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 1995;252(1):11-4.
36. Georgalas C, Garas G, Hadjihannas E, Oostra A. Assessment of obstruction level and selection of patients for obstructive sleep apnoea surgery: An evidence-based approach. J Laryngol Otol 2010;124(1):1-9.
37. McLean HA, Urton AM, Driver HS, Tan AK, Day AG, Munt PW, et al. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J 2005;25(3):521-7.
38. McNicholas WT. The nose and OSA: Variable nasal obstruction may be more important in pathophysiology than fixed obstruction. Eur Respir J 2008;32(1):3-8.
|
|
|||||||||||||||||||||||||||||||||||||||

 |
 |