 |
 |
AbstractBackground and Objectives With the growing acknowledgment of age-related vestibular impairments, consensus diagnostic criteria for presbyvestibulopathy (PVP) have been recently published. The PVP criteria retains its objectivity with video head impulse test (vHIT), rotatory chair test (RCT) and caloric test. These objective tests share testing principles, but targeted frequencies vary across the tests. The PVP criteria are applicable for subjects of age over 60. However, there are different age cut-offs for older male in different areas. The purpose of the present study was to explore whether the PVP diagnostic criteria were appropriate for early elderly (Eel) patients and to identify the most sensitive test for age-related loss of vestibular function.
Subjects and Method Eligible patients, aged 60 years or older, complained of dizziness for at least 3 weeks, and have undergone at least one of the followings: vHIT, RCT, or caloric test. We selected two groups based on age: the Eel group (aged >60 years but <65 years) and the late elderly (Lel) group (aged Ōēź75 years).
Results The vestibulo-ocular reflex (VOR) gains differed significantly between the Eel and Lel groups for vHIT and caloric gain test. No Eel patient met the PVP criteria but 26.1% of Lel patients met the criteria for vHIT, and the proportions of such patients in the two groups differed significantly.
IntroductionWorldwide, populations are aging. South Koreans aged 65 years or older will constitute 37% of the population in 2045 and 47% in 2067 [1]. Aged patients often complain of dizziness. Age-related vestibular impairment attains 50% in those aged over 60 years, compromising daily activities and quality of life [2,3]. Such impairment is more common than vision loss (presbyopia) and hearing loss (presbycusis) the levels of which are 15% and 26% in those aged over 70 years [4]. With age, histological decreases in the levels of vestibular hair cells and otoconia are evident [5-7]; reduction of vestibulo-ocular reflex (VOR) in slow harmonic acceleration (SHA) is observed [8]; VOR processing and velocity storage decrease [9]; the video head impulse test (vHIT) gain falls [10]; and otolith dysfunctions become more apparent [11].
As the growing acknowledgment of age related vestibular impairments and due to the recent development/improvement of vestibular function tests (VFT), consensus diagnostic criteria for presbyvestibulopathy (PVP) have been published. Objective means in this diagnostic criteria are used to detect mild, bilateral, peripheral vestibular hypofunction that is not falls within a diagnostic criterion for other diseases. PVP is objectively measured by the vHIT, or via assessment of SHA or the caloric response (Table 1). These objective tests share common testing principles of VOR measurement, but targeted frequencies of measurements, sensitivity and specificity for VOR varies across the test modalities. Thus, for now, it is unclear which test method is suitable for accessing the age related VOR decline. The PVP criteria are (strictly) applicable only to those aged over 60 years; this is the United Nations cutoff for older adults in 2019 [11]. However, there are different age cut off for old aged male in different areas. For example, the Korean government categorized old age male of 65 year or older for Elderly Welfare Act. As the life expectancies of developed nations get higher, due to the development of medical technology and enriched environmental resources, people of age range between 60 to 70 donŌĆÖt seem to face a lot of difficulties in social activities and donŌĆÖt consider themselves as ŌĆśoldŌĆÖ in several nations.
Of patients who visited our tertiary referral hospital with chronic dizziness, we compared the ŌĆśearlyŌĆÖ elderly (Eel) and ŌĆślateŌĆÖ elderly (Lel). We averaged the objective VOR values of the three tests to define the percentages within the objective PVP criteria; we sought the test that revealed the greatest difference. The purposes of these comparisons were to explore whether the PVP diagnostic criteria were appropriate for Eel patients and to identify the test that most sensitively detected age-related loss of vestibular function in subjects with chronic dizziness.
Subjects and MethodsPatients and VFTsA retrospective study was conducted with patients who visited the ENT outpatient department of Dankook University Hospital from January 2011 to December 2021. This study was approved by Institutional Review Board of Dankook University Hospital (2022-07-007). Eligible patients were aged 60 years or older and complained of dizziness that had persisted for at least 3 weeks; all underwent at least one of the following VFTs: The vHIT, the rotatory chair test (RCT), or the caloric test. We selected two groups based on an age: an Eel group (aged >60 years but <65 years) and an Lel group (aged Ōēź75 years; including thirty subjects from the oldest patient). This age criteria was determined based on other clinical researches [12,13] and statistics data from Korean government which classified ŌĆśolder adultsŌĆÖ into ŌĆśaged Ōēź65 year but <70 years,ŌĆÖ ŌĆśaged Ōēź70 year but <75,ŌĆÖ and ŌĆśaged Ōēź75 years.ŌĆÖ [14] Patients diagnosed with MeniereŌĆÖs disease, benign paroxysmal positional vertigo, vestibular neuritis or otitis media were excluded and also patients who were taking any medication such as antidepressants or anti-Alzheimers were excluded. The imaging studies with MR or CT was performed to exclude central pathologies.
To evaluate vestibular function, we performed the vHIT, RCT, and/or the caloric test. The vHIT employed an ICS Impulse device (GN Otometrics, Taastrup, Denmark). Subjects were seated while wearing goggles and facing a wall 1 m distant that bore a small LED target. The subject was instructed to stare at the target while an examiner, standing behind the subject, rotated his/her head in a brief, abrupt, and unpredictable manner. To test all semicircular canals, about 20 impulses in each direction were manually delivered at small amplitudes (5┬║-15┬║) but high peak velocities (150-250┬║/s). But only the VOR gain of both lateral semicircular canals were finally used. As for objective PVP classification using VOR gain of vHIT, patient was classified into objective PVP when both ear gains are within the criteria or one ear gain is within the criteria and the other ear is normal range. As for quantitative analysis of VOR gain, average gain of both lateral semicircular canal was used. The binaural, bithermal caloric test used 30┬░C (cool) and 44┬░C (warm) water. Nystagmus was recorded using a System 2000 videonystagmography (VNG) device (Micromedical, Chatham, IL, USA). The System 2000 Rotation Chair System (Micromedical) was used to record eye movements. The slow-phase velocities of eye movement (on the caloric test) were measured on both sides and compared between the Eel and Lel groups (Fig. 1). The four respective caloric gains were summed and compared (Table 1B). We studied the measured VOR gains or the VNG-measured slowphase velocity of eye movement (these are termed the ŌĆśVOR gainŌĆÖ and ŌĆścaloric test gainŌĆÖ below).
StatisticsThe results were expressed as means┬▒standard deviations (SDs) and compared using GraphPad Prism software (La Jolla, CA, USA) or SPSS software (IBM Corp., Armonk, NY, USA). The independent t-test and the chi-squared test were employed to compare demographic characteristics. Significant differences in the VFT results of the Eel and Lel groups were sought using the independent t-test, Mann-Whitney U-test, Fisher test, and the chi-squared test (as appropriate). A p-value<0.05 was considered statistically significant.
ResultsDemographic characteristicsThe 60 included patients were divided into Eel (n=30) and Lel (n=30) groups. We compared the sex ratio, age, and hypertension (HTN) and diabetes mellitus (DM) status of the two groups; all differed significantly. The Eel group was predominantly male but the Lel group predominantly female; the difference was significant (chi-squared test p=0.020). The average Eel group age was 61.3 years and that of the Lel group 81.5 years (independent t-test p<0.001). As expected, the Lel group reported significantly more histories of HTN (chi-squared test p=0.001) and DM (chi-squared test p=0.044) (Table 2). We considered that DM might affect the VOR gain based on several prior reports [15,16]. Thus, we compared the VORs of Lel subjects with and without DM, and of Eel and Lel subjects without DM (Table 3).
Differences in the VOR gains of the three VFT tests between the Eel and Lel groupsVOR gains were measured based on the objective PVP diagnostic criteria (ŌĆśCriteria BŌĆÖ) (Table 1). There are several purposes for comparing the VOR gains of the three tests between the Eel and Lel groups. Firstly, given the average age difference of about 20 years, this would reveal decreases in VOR gains. Second, this would also identify the test revealing the greatest differences; this test would optimally evaluate PVP. Last, the comparison might suggest major differences between the groups; the Eel group might require additional diagnostic measures. Indeed, the VOR gains differed significantly between the Eel and Lel groups on the vHIT (Mann-Whitney test p<0.001) and caloric gain test (Mann-Whitney test p=0.036) (Fig. 1). The average vHIT VOR gain of the Eel group was almost 1.0 but that of the Lel group only 0.77; the caloric test gains were about 30 (Eel) and 20 (Lel). The average RCT VOR gain was lower in the Lel than the Eel group, but statistical significance was not attained (Mann-Whitney test p=0.143). Thus, age-related decreases in VOR gain are evident in those with chronic dizziness and the vHIT and caloric tests detect these rather sensitively. As mentioned above, as we thought it possible that DM could affect the VOR gain, we performed a subgroup analysis by non-DM status. The results were similar to those described above. Significant decreases in the VOR gains of the non-DM Lel group compared to the non-DM Eel group were identified by both the vHIT (independent t-test p<0.001) and the caloric gain test (Mann-Whitney test p=0.019) but not by the RCT (Mann-Whitney test p=0.140) (Table 3). We also compared the VOR gains between DM and non-DM patients in the Lel group; we found no difference. As expected, the VOR gains revealed by the vHIT (Mann-Whitney test p=0.968), RCT (Mann-Whitney test p=0.909), and caloric test (Mann-Whitney test p=0.291) did not differ between DM and non-DM patients in the Lel group (Table 4).
Proportions of patients meeting the PVP objective criteria in the Eel and Lel groupsThe lack of any difference in VOR gain between DM and non-DM patients in the Lel group and the fact that aging inevitably increases the HTN and DM rates indicated that our measurements of the proportions of patients who met the PVP objective criteria were reasonable. No Eel patient met the PVP criteria but 26.1% Lel patient met the criteria on the vHIT, and the proportions of such patients in the two groups differed significantly (Fisher test p=0.009). On the other hand, even though somewhat fewer patients met the objective PVP criteria on the RCT and caloric test in the Eel compared to the Lel group, the differences were not significant (RCT: Fisher test p=0.414; Caloric test: Fisher test p=0.391) (Table 5). Of the two VFT tests, the results of which differed significantly between the Eel and Lel groups, use of the vHIT to evaluate the objective PVP criteria seemed to discriminate, optimally, the Eel and Lel groups. The vHIT may thus be the test of choice when evaluating age-related changes in VOR gain. Finally, as only one of the three tests must meet the PVP, we assessed how many subjects met the PVP ŌĆśCriteria BŌĆÖ in each group. The Eel proportion was 20% and the Lel proportion over 50% (53.3%); the difference was significant (chi-squared test p=0.007) (Table 5).
DiscussionSubjects with chronic dizziness evidenced reduced VOR gains on the vHIT and caloric test by age; we compared the average gains of the Eel and Lel groups. In terms of the objective PVP diagnostic criteria, a much higher proportion of the Lel group met the criteria. This may suggest that Eel subjects should be considered to differ from Lel subjects, and should perhaps be diagnosed using modified criteria. Given the significance of both the average VOR difference and the proportional difference in terms of meeting the PVP criteria, the vHIT may be optimal when evaluating age-related changes.
To meet the PVP diagnostic criteria, Criteria A through D must be met (Table 1). As mentioned above, Criteria B objectively diagnose mild vestibular hypofunction and Criterion C considers age. Criterion D rules out other vestibular diseases. We have not discussed Criteria A. To fulfill the Criteria A, at least two chronic symptoms among postural imbalance/unsteadiness, gait disturbance, chronic dizziness, and recurrent falls must be present. As we included only subjects with chronic dizziness only one out of four symptom is identified and one more symptom must be confirmed to meet Criteria A. Thus, for now, even if patients fulfill Criteria B-D, they cannot be diagnosed with PVP. Furthermore, as recurrent falls and gait disturbances are rare in Eel populations, the PVP diagnostic rate will be low especially in Eel group.
The vHIT is a recent introduction in balance clinics. It has been readily accepted; many scholars have reported that that the data are valuable and that the vHIT should be included in the test battery for vestibular examination. This is very good news; the test does not (excessively) distress subjects when carefully performed [17]. The vHIT revealed the greatest difference between the Eel and Lel groups. Therefore, if using only one test, the vHIT is perhaps optimal. However, as the vHIT was performed more commonly than the RCT and the caloric test in present study (Table 5), a further study with more subjects or study with all subjects performing all three tests would be helpful.
There are several limitations of the present study. First, we analyzed the same subjects at different time points, and there were only 30 subjects in each group; the study design was not optimal. Second, as did an earlier work on the diagnostic criteria for PVP, we focused on diagnosis, not therapy. It is not clear that PVP can be managed either pharmacologically or via vestibular rehabilitation. We did not seek differences in therapeutic efficacies between the two groups; it is possible that the groups have different diseases. Lastly, even though several studies have observed the correlation between DM and vestibular dysfunction [18,19], we found that the effect of DM on VOR gain was not statistically significant in the subgroup analysis. On the otherhand, subgroup analysis of hypertension and dizziness [20] was not possible because that most of Lel patients had history of hypertension and taking medication for it. Thus, these points have to be considered before the interpretation of the data of the present study.
Despite the limitations, the two facts that the Eel and Lel groups differed markedly in terms of the VOR gains, and the vHIT may optimally reveal age-related mild vestibulopathy should not be underestimated and must be considered in planning future studies.
ACKNOWLEDGMENTSThis research was supported by the Basic Science Research Program through the National Foundation of Korea (NRF-2021R1I1A3047407); by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1A6A1A03043283); by a grant from a Creative Materials Discovery Program through the National Research Foundation (2019M3D1A1078943) funded by the National Research Foundation of Korea (NRF); and by the Leading Foreign Research Institute Recruitment Program through the NRF, funded by the Ministry of Science and ICT (MSIT) (NRF-2018K1A4A3A02060572).
NotesAuthor Contribution Conceptualization: Jaeil Kim, Ji-Eun Choi, Jae Yun Jung, Min Young Lee. Data curation: Ji Hyeok Choi, Hyoung-Sik Park, Min Seok Song. Formal analysis: all authors. Methodology: Ji-Eun Choi, Jae Yun Jung. Supervision: Jaeil Kim, Ji-Eun Choi, Jae Yun Jung, Min Young Lee. WritingŌĆöoriginal draft: Ji Hyeok Choi. WritingŌĆöreview & editing: Jaeil Kim, Ji-Eun Choi, Jae Yun Jung, Min Young Lee. Fig.┬Ā1.The average VOR gains measured by the three different tests (the vHIT, RCT, and caloric test) and the differences between early and late elderly patients. A: The average vHIT gain of early elderly patients was very close to normal (1.0), The average vHIT gain of late elderly patients was lower than 0.8. The difference was significant. B: The average RCT gain of the early elderly was somewhat higher than that of the late elderly, but statistical significance was not attained. C: The average caloric gain was lower in the late than the early elderly, with statistical significance. *p<0.05. VOR, vestibulo-ocular reflex; vHIT, video head impulse test; RCT, rotatory chair test. 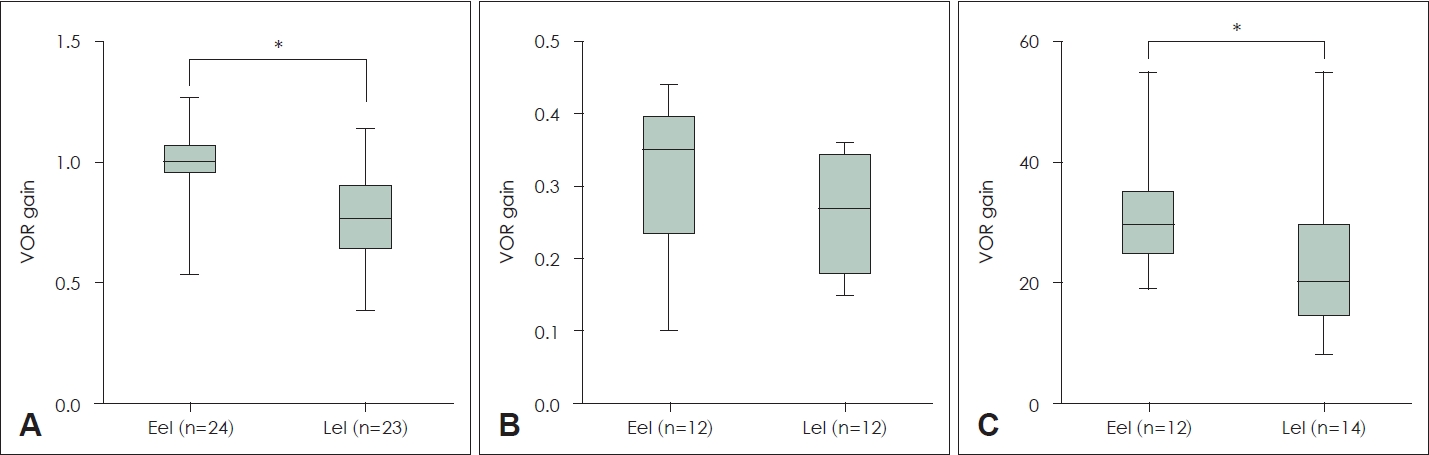
Table┬Ā1.Objective diagnostic criteria of presbyvestibulopathy Table┬Ā2.Demographic characteristics of the patients
Table┬Ā3.The average VOR gains measured in three different ways (using the vHIT, the RCT, and the caloric test) and the differences between Eel and Lel non-DM patients
Table┬Ā4.The average VOR gains measured using three different tests (the vHIT, RCT, and caloric test) and the differences between DM and non-DM patients in the Lel group
Table┬Ā5.Vestibular function tests results meeting the objective diagnostic criteria for presbyvestibulopathy
REFERENCES1. Wikimedia-Commons WCc. South korea population pyramid 1960-2020. Wikimedia Commons, the free media repository 2021 [cited 2022 Sep 6]; Available from: URL: https://commons.wikimedia.org/wiki/File:South_korea_population_pyramid_1960-2020.gif.
2. Agrawal Y, Zuniga MG, Davalos-Bichara M, Schubert MC, Walston JD, Hughes J, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol 2012;33(5):832-9.
3. Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: Data from the national health and nutrition examination survey, 2001-2004. Arch Intern Med 2009;169(10):938-44.
4. Dillon CF, Gu Q, Hoffman HJ, Ko CW. Vision, hearing, balance, and sensory impairment in Americans aged 70 years and over: United States, 1999-2006. NCHS Data Brief 2010;31:1-8.
5. Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: Past, present and future. Clin Neurophysiol 2010;121(5):636-51.
6. Semenov YR, Bigelow RT, Xue QL, du Lac S, Agrawal Y. Association between vestibular and cognitive function in U.S. adults: Data from the national health and nutrition examination survey. J Gerontol A Biol Sci Med Sci 2016;71(2):243-50.
7. Rosenhall U. Degenerative patterns in the aging human vestibular neuro-epithelia. Acta Otolaryngol 1973;76(1-6):208-20.
8. Serrador JM, Lipsitz LA, Gopalakrishnan GS, Black FO, Wood SJ. Loss of otolith function with age is associated with increased postural sway measures. Neurosci Lett 2009;465(1):10-5.
9. Tuunainen E, Poe D, J├żntti P, Varpa K, Rasku J, Toppila E, et al. Presbyequilibrium in the oldest old, a combination of vestibular, oculomotor and postural deficits. Aging Clin Exp Res 2011;23(5-6):364-71.
10. Jacobson GP, McCaslin DL, Grantham SL, Piker EG. Significant vestibular system impairment is common in a cohort of elderly patients referred for assessment of falls risk. J Am Acad Audiol 2008;19(10):799-807.
11. Strupp M, Kim JS, Murofushi T, Straumann D, Jen JC, Rosengren SM, et al. Bilateral vestibulopathy: Diagnostic criteria consensus document of the classification committee of the B├Īr├Īny Society. J Vestib Res 2017;27(4):177-89.
12. Hishikawa N, Fukui Y, Sato K, Kono S, Yamashita T, Ohta Y, et al. Characteristic features of cognitive, affective and daily living functions of late-elderly dementia. Geriatr Gerontol Int 2016;16(4):458-65.
13. Croci DM, Sherrod B, Alvi MA, Mummaneni PV, Chan AK, Bydon M, et al. Differences in postoperative quality of life in young, early elderly, and late elderly patients undergoing surgical treatment for degenerative cervical myelopathy. J Neurosurg Spine 2022;37(3):339-49.
14. Statistics Korea. 2021ļģä Statistics on the elderly. Daejeon: Statistics Korea; 2021.
15. Bakhshizadeh R, Rahbar N, Akbari M, Hashemi Madani N. The study of the results of video head impulse test (vHIT) in type II diabetic patients with and without neuropathy. Razi Journal of Medical Sciences 2019;25(12):96-103.
16. Teggi R, Trimarchi M, Gatti O, Fornasari F, Bussi M. Decrease of horizontal canal vestibulo-oculomotor reflex gain in the elderly with dysequilibrium without lifetime vertigo. ORL J Otorhinolaryngol Relat Spec 2017;79(3):178-84.
17. Yoo MH. Clinical application of video head impulse test in acute vestibular syndrome. Korean J Otorhinolaryngol-Head Neck Surg 2020;63(1):3-13.
18. Ward BK, Wenzel A, Kalyani RR, Agrawal Y, Feng AL, Polydefkis M, et al. Characterization of vestibulopathy in individuals with type 2 diabetes mellitus. Otolaryngol Head Neck Surg 2015;153(1):112-8.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |